Department of Medical Oncology & Experimental Therapeutics, City of Hope Comprehensive Cancer Center, Duarte, California
Case Report
Renal cell carcinoma (RCC) has a multitude of histologic subtypes. The most prevalent subtype is clear cell RCC, followed by papillary RCC and chromophobe RCC.1 Together, these subtypes make up about 90% of all RCC cases, but several extremely rare histologies make up an even smaller proportion of the disease, including collecting duct carcinoma, renal medullary carcinoma, and Xp11 translocation RCC.1 One histologic subtype that has garnered limited attention is mucinous tubular and spindle cell carcinoma (MTSCC) of the kidney. This variant makes up less than 1% of all RCC cases, and fewer than 100 cases have been reported in the literature.2 The World Health Organization tumor classification listed MTSCC as a distinct entity in 2004 because of its unique immunohistochemical, histologic, and molecular features.3 Microscopic features of MTSCC include a tubular structure, mucinous stroma, and spindle-shaped cells. The clinical presentation of MTSCC varies, but most patients are incidentally diagnosed through imaging modalities ordered for another indication. Specifically, MTSCC can be identified on computed tomographic images primarily by its hypovascular features.4 This subtype is exceedingly rare in men, with women being 4 times more likely to receive a diagnosis of MTSCC.5
Although MTSCC is classically described as an indolent disease, high-risk features such as sarcomatoid components are associated with a higher risk of metastasis and present with an aggressive clinical course. Here, we present the case of a patient with MTSCC with sarcomatoid features whose disease was refractory to multiple lines of systemic treatment. Written informed consent was obtained from the patient and her primary oncologist for publication of this case report.
Presentation
Abbreviations
MTSCC mucinous tubular and spindle cell carcinoma
RCC renal cell carcinoma
The patient is a 67-year-old woman diagnosed with MTSCC in 2014 who underwent a left-sided, open radical nephrectomy. Surgical pathology showed pT2a RCC, Fuhrman grade 3 MTSCC, with a sarcomatoid component of 50%. Despite the initial diagnosis, the patient remained on active surveillance for almost 3 years, and serial imaging showed no evidence of disease activity (Figure 1). In 2017, however, imaging showed local disease progression in the renal fossa and omental nodules suggestive of disease activity (Figure 2a), which prompted a computed tomography–guided biopsy of the left renal fossa. Pathology confirmed recurrent disease, for which the patient underwent percutaneous cryoablation of the omental nodule and left renal fossa. Immediately after percutaneous cryoablation, imaging showed stability of these lesions, and magnetic resonance imaging of the brain was unremarkable.
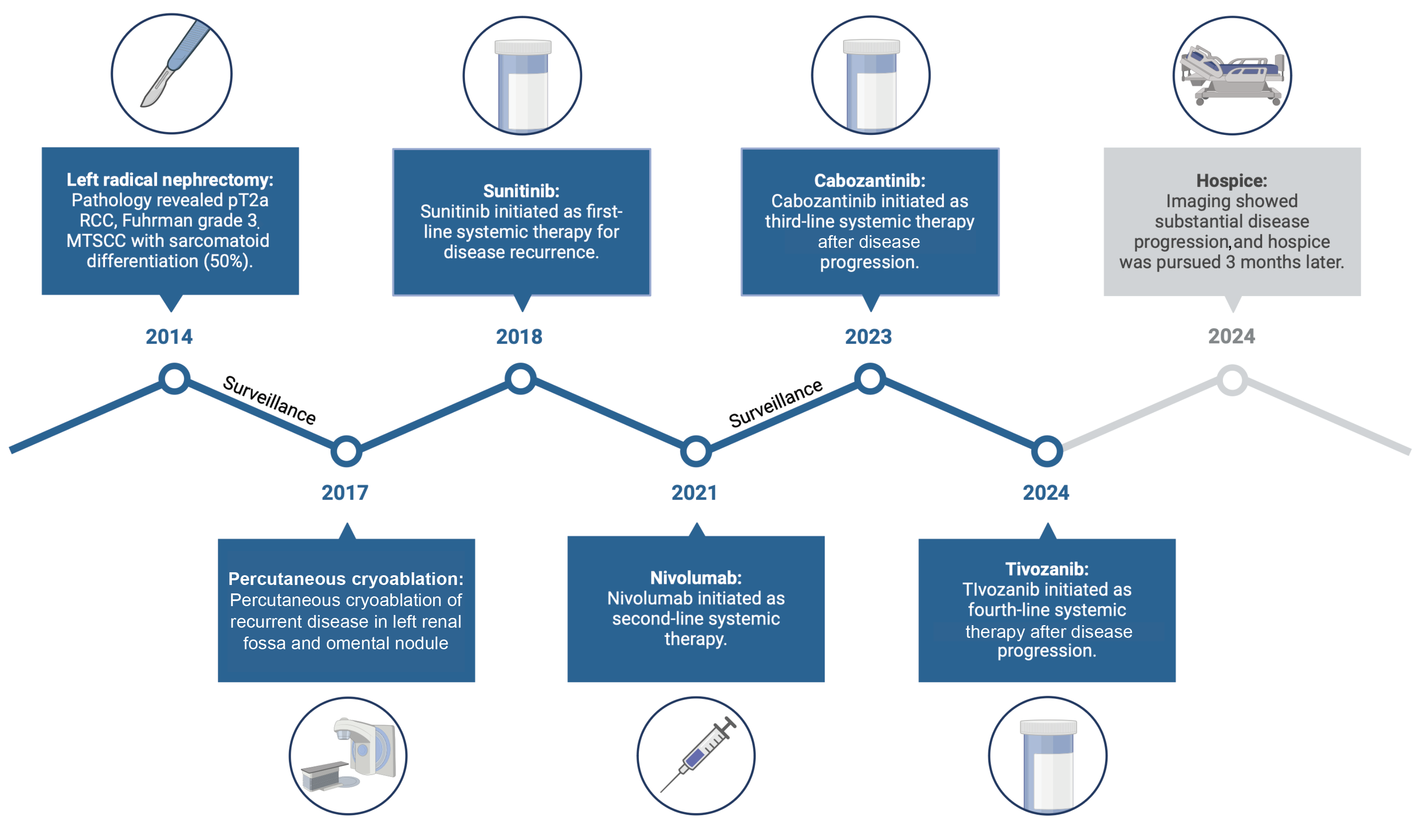
Figure 1. Timeline of therapies
Abbreviations: MTSCC, mucinous tubular spindle cell carcinoma; PCA, percutaneous cryoablation; RCC, renal cell carcinoma.
During follow-up in early 2018, imaging showed recurrence, with multiple peritoneal nodules. The patient began sunitinib in early 2018 and had a dosage reduction to 37.5 mg daily (2 weeks on, 1 week off) 2 months later. Eighteen months into treatment with sunitinib, the patient was hospitalized for aspiration pneumonia and urinary tract infection, which resulted in a 5-month treatment holiday. When the patient restarted sunitinib, the dosage was further reduced to 25 mg daily (2 weeks on, 1 week off), and imaging in 2020 showed stability, but sunitinib was continued only an additional year when imaging ultimately revealed disease progression. Subsequently, the patient started nivolumab as second-line systemic therapy in 2021. Unfortunately, treatment was discontinued after 1 cycle because of clinically significant immune-related colitis, for which the patient received prednisone and was subsequently placed on surveillance until 2023. At that time, new mediastinal lymph nodes showed evidence of disease progression, and cabozantinib was initiated. The patient received cabozantinib at 20 mg daily for 3 months, and the dosage was increased to 40 mg daily for better disease control after the drug demonstrating good tolerability (Figure 2b). The dosage was reduced to 20 mg daily 4 months later, however, because of drug-related toxicity (ie, colitis). In early 2024, a computed tomographic scan showed an increase in the size and extent of lymph nodes in the mediastinal region. Endobronchial and supraclavicular node biopsies were performed, the former confirming recurrent MTSCC. The patient was prescribed tivozanib in 2024 and treated for 3 months, but when imaging showed substantial disease progression, the patient chose to pursue hospice.
Discussion
We present the case of a patient with aggressive MTSCC with sarcomatoid differentiation, as evidenced by the disease’s refractoriness to multiple lines of systemic therapy. The World Health Organization tumor classification listed MTSCC as an independent entity in 2004.6 It is a rare histopathologic entity, accounting for less than 1% of all RCC cases.7 Although it was previously considered a subvariant of papillary RCC, genomic and immunohistochemical analysis has proven it to be an independent entity, establishing it as a clinically heterogeneous variant.7
The MTSCC variant predominantly affects women 13 to 82 years of age, with a median age of incidence of 53 years.8 As in conventional RCC, large tumors can present with flank pain, a palpable mass, and hematuria, but most cases are discovered incidentally. On imaging, MTSCC is described as an ovoid, well-demarcated, exophytic mass more commonly found in the renal cortex.8 No specific dimensions have been attributed to MTSCC because tumors as small as 1 cm and as large as 18 cm have been described. Tumors larger than 5 cm have also been described to have a heterogeneous enhancement pattern on imaging.8 Tumors with classic morphology and no high-risk features are typically benign and amenable to partial or radical nephrectomy with serial follow-up. Tumors with high-risk features, however, such as higher mitotic rates, nuclear atypia, and sarcomatoid features, are more likely to metastasize. Because of the rare nature of this condition, treatment guidelines specifying drug selection are lacking, although some authors have described responses to targeted therapy. For instance, Larkin et al9 described a case of MTSCC that showed response to sunitinib.
Histologically, these tumors consist of tubular elements and spindle cells surrounded by mucinous stroma. Although MTSCC was initially conceived as an indolent entity, cases with sarcomatoid differentiation have been classified as high grade. These variants elicit a higher cell proliferation rate, marked cellular atypia, tumoral necrosis, and a more aggressive disease course. Immunohistochemical positivity to CK7 and α-methylacyl-CoA racemase suggests that this tumor originates from the proximal nephron.10 Peckova et al11 performed genetic analysis on the most extensive series of MTSCC cases and described chromosomal losses in chromosomes 1, 4, 6, 8, 9, 13, 14, 15, and 22. Furthermore, they demonstrated MTSCC’s distinct identity from papillary RCC by performing fluorescence in situ hybridization–based analysis and showing a lack of gains in chromosomes 7 and 17 and losses of chromosome Y, which are characteristic of papillary RCC.12 Genetic and immunohistochemical analysis has characterized MTSCC as a molecularly heterogeneous entity.
Conclusions
Although MTSCC remains an underrepresented RCC variant, advances in techniques such as genomic characterization and fluorescence in situ hybridization–based analysis have demonstrated the heterogeneous nature of this condition. It was classically described as a slow-growing, indolent variant, but case descriptions have shown key histopathologic characteristics associated with a more aggressive disease course. This case highlights the heterogeneous nature and potential clinical course of MTSCC, demonstrating how crucial it is to perform extensive histopathologic characterization to identify key high-risk features necessary for optimal decision-making.
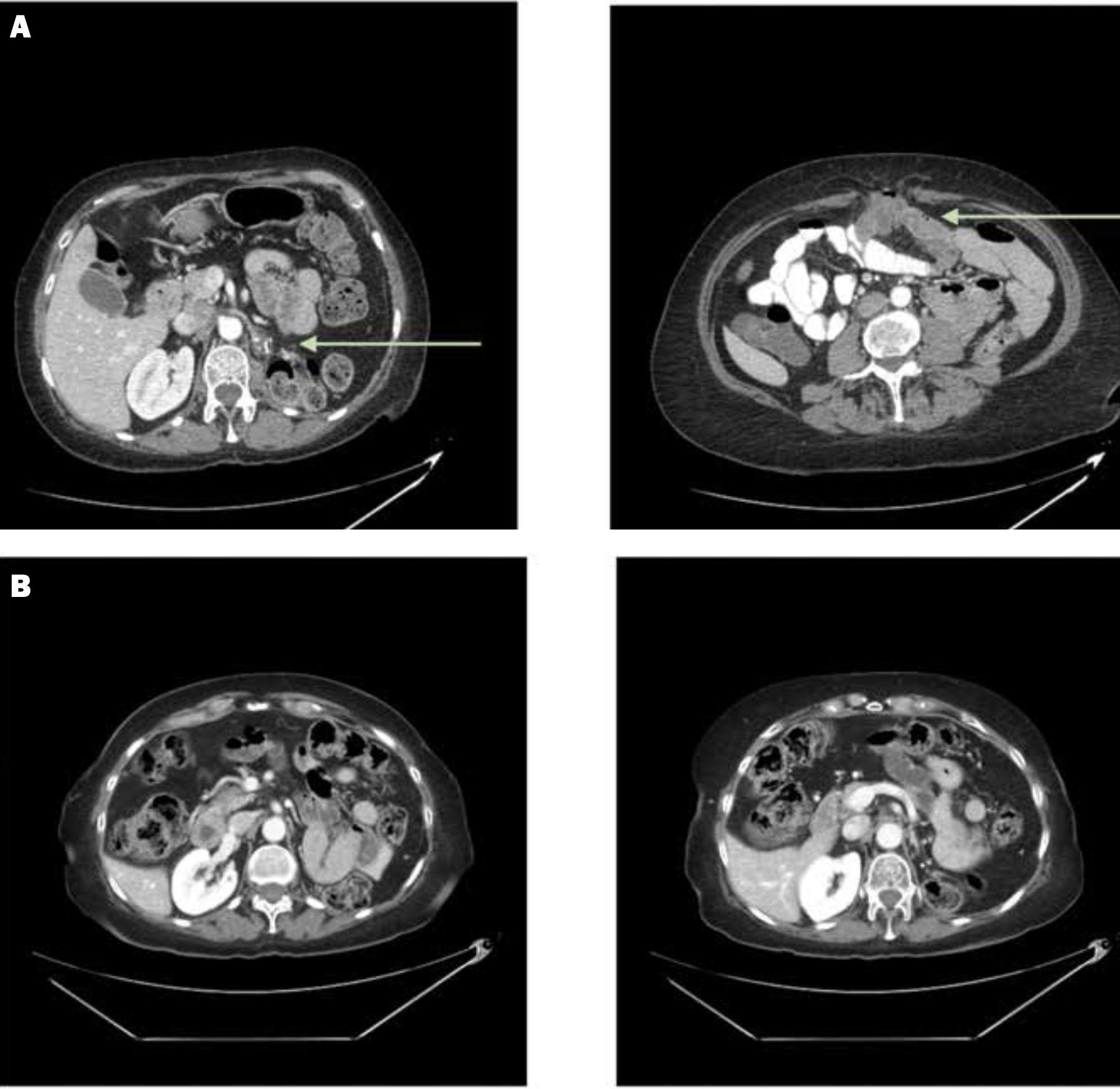
Figure 2. Computed tomographic scans of the abdomen and pelvis highlight (A) a nodule in the kidney fossa and omental disease at baseline (arrows) and (B) response after targeted therapy and checkpoint inhibition
References
1. Salgia NJ, Zengin ZB, Pal SK, Dizman N. Renal cell carcinoma of variant histology: new biologic understanding leads to therapeutic advances. Am Soc Clin Oncol Educ Book. 2024;44(3):e438642. doi:10.1200/EDBK_438642
2. Ali RH, Ali RM, Ali SM, et al. Mucinous tubular and spindle cell carcinoma: a case report. World Acad Sci J. 2024;6(2):1-4. doi:10.3892/wasj.2024.229
3. Bajpai M, Pooja S, Tyagi M, Pathre A. Mucinous spindle and tubular renal cell cancer: a rare variant of renal cell cancer. J Cancer Res Ther. 2022;18(4):1168. doi:10.4103/jcrt.jcrt_99_21
4. Ling C, Tan R, Li J, Feng J. Mucinous tubular and spindle cell carcinoma of the kidney: a report of seven cases. BMC Cancer. 2023;23:815. doi:10.1186/s12885-023-11252-z
5. Ren Q, Wang L, Al-Ahmadie HA, et al. Distinct genomic copy number alterations distinguish mucinous tubular and spindle cell carcinoma of the kidney from papillary renal cell carcinoma with overlapping histologic features. Am J Surg Pathol. 2018;42(6):767-777. doi:10.1097/PAS.0000000000001038
6. Eble JN, Sauter G, Epstein J, Sesterhenn I. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. International Agency for Research on Cancer; 2004. Accessed July 8, 2024. https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Pathology-And-Genetics-Of-Tumours-Of-The-Urinary-System-And-Male-Genital-Organs-2004
7. Nathany S, Monappa V. Mucinous tubular and spindle cell carcinoma: a review of histopathology and clinical and prognostic implications. Arch Pathol Lab Med. 2020;144(1):115–118. doi:10.5858/arpa.2017-0506-RS
8. Zhao M, He XL, Teng XD. Mucinous tubular and spindle cell renal cell carcinoma: a review of clinicopathologic aspects. Diagn Pathol. 2015;10(1):168. doi:10.1186/s13000-015-0402-1
9. Larkin J, Fisher R, Pickering L, et al. Metastatic mucinous tubular and spindle cell carcinoma of the kidney responding to sunitinib. J Clinic Oncol. 2010;28(28):e539-e540. doi:10.1200/JCO.2010.30.1457
10. Paner GP, Srigley JR, Radhakrishnan A, et al. Immunohistochemical analysis of mucinous tubular and spindle cell carcinoma and papillary renal cell carcinoma of the kidney: significant immunophenotypic overlap warrants diagnostic caution. Am J Surg Pathol. 2006;30(1):13. doi:10.1097/01.pas.0000180443.94645.50
11. Peckova K, Martinek P, Sperga M, et al. Mucinous spindle and tubular renal cell carcinoma: analysis of chromosomal aberration pattern of low-grade, high-grade, and overlapping morphologic variant with papillary renal cell carcinoma. Ann Diagn Pathol. 2015;19(4):226-231. doi:10.1016/j.anndiagpath.2015.04.004
12. Cossu-Rocca P, Eble JN, Delahunt B, et al. Renal mucinous tubular and spindle carcinoma lacks the gains of chromosomes 7 and 17 and losses of chromosome Y that are prevalent in papillary renal cell carcinoma. Mod Pathol. 2006;19(4):488-493. doi:10.1038/modpathol.3800565
Article Information
Published: September 13, 2024.
Conflict of Interest Disclosures: The authors have no conflicts to disclose.
Funding/Support: None.
Author Contributions: Salvador Jaime-Casas, Daniela V. Castro, Ruchi Agarwal (concept and design); Yu Jun Li, Jaya Goud (acquisition, analysis, and interpretation of data); Ruchi Agarwal, Daniela V. Castro (drafting of the article); Salvador Jaime-Casas, Yu Jun Li (administrative, technical, or material support); Salvador Jaime-Casas (supervision).
Data Availability Statement: The data that support the findings of this case report are openly available in the references listed.
Citation: Agarwal R, Castro DV, Li YJ, Goud J, Jaime-Casas S. Response to targeted therapy and checkpoint inhibition in a patient with mucinous tubular and spindle cell carcinoma: a case report and literature review. Rev Urol. 2024;23(3):e71-e75.
Corresponding author: Salvador Jaime-Casas, MD, City of Hope Comprehensive Cancer Center, 1500 E Duarte Rd, Duarte, CA 91010 (sjaimecasas@coh.org)