Carolina Urologic Research Center and Atlantic Urology Clinics, Myrtle Beach, South Carolina
Introduction
Non–muscle-invasive bladder cancer (NMIBC) encompasses approximately three-fourths of new bladder cancer diagnoses and is currently the fourth-most diagnosed cancer in men in the United States.1-3 Intravesical bacille Calmette-Guérin has been the gold-standard treatment for patients with high-risk NMIBC following endoscopic resection. Despite the well-described efficacy of this therapy, however, disease recurrence and progression rates remain a clinical concern; similarly, treatment intolerability and the unpredictability of bacille Calmette-Guérin availability and accessibility have led to investigation of alternative treatment modalities.1
Current guidelines state that the treatment for bacille Calmette-Guérin–unresponsive bladder cancer is radical cystectomy.4 Given the substantial morbidity associated with this procedure, even within high-volume centers of excellence, many patients reject this option. Further, a substantial number of patients are not appropriate candidates for the procedure given its surgical and anesthesia-related risks. Bladder-preservation strategies have therefore been evolving rapidly.1
Abbreviations
BladderGATE Atezolizumab and BCG in High Risk BCG naïve Non-muscle Invasive Bladder Cancer Patients
CIS carcinoma in situ
CREST A Study of Sasanlimab in People With Non-muscle Invasive Bladder Cancer
KEYNOTE-676 Efficacy and Safety of Pembrolizumab in Combination With Bacillus Calmette-Guerin in High-Risk Non-Muscle Invasive Bladder Cancer
mAb monoclonal antibody
NMIBC non–muscle-invasive bladder cancer
PD-1 programmed cell death 1 protein
PD-L1 programmed cell death 1 ligand 1
POTOMAC Assessment of Efficacy and Safety of Durvalumab Plus BCG Compared to the Standard Therapy With BCG in Non-muscle Invasive Bladder Cancer
Checkpoint proteins (eg, programmed cell death 1 protein [PD-1], programmed cell death 1 ligand 1 [PD-L1]) are an integral part of both the immune system, wherein they reduce the proliferation of T cells, and urothelial neoplastic cells. As a result, these checkpoint proteins allow tumor cells to evade an apoptotic immune response, which may in turn promote proliferation of neoplastic cells. By blocking these proteins, immune checkpoint inhibitors can help the immune system better recognize cancer cells and improve cell killing rates.5 Despite their clinical efficacy, however, immune checkpoint inhibitors can induce immune-related adverse events—for example, peripheral tolerance to autoantigens, resulting in autoantibody formation—limiting their use in many patients.6 Nevertheless, the enhanced immune checkpoint expression shown in patients with bacille Calmette-Guérin–unresponsive disease as well as the activity of immune checkpoint inhibitors in advanced bladder cancer and other metastatic diseases provide the rationale for testing immune checkpoint inhibitors in patients with bacille Calmette-Guérin–naive NMIBC.7,8
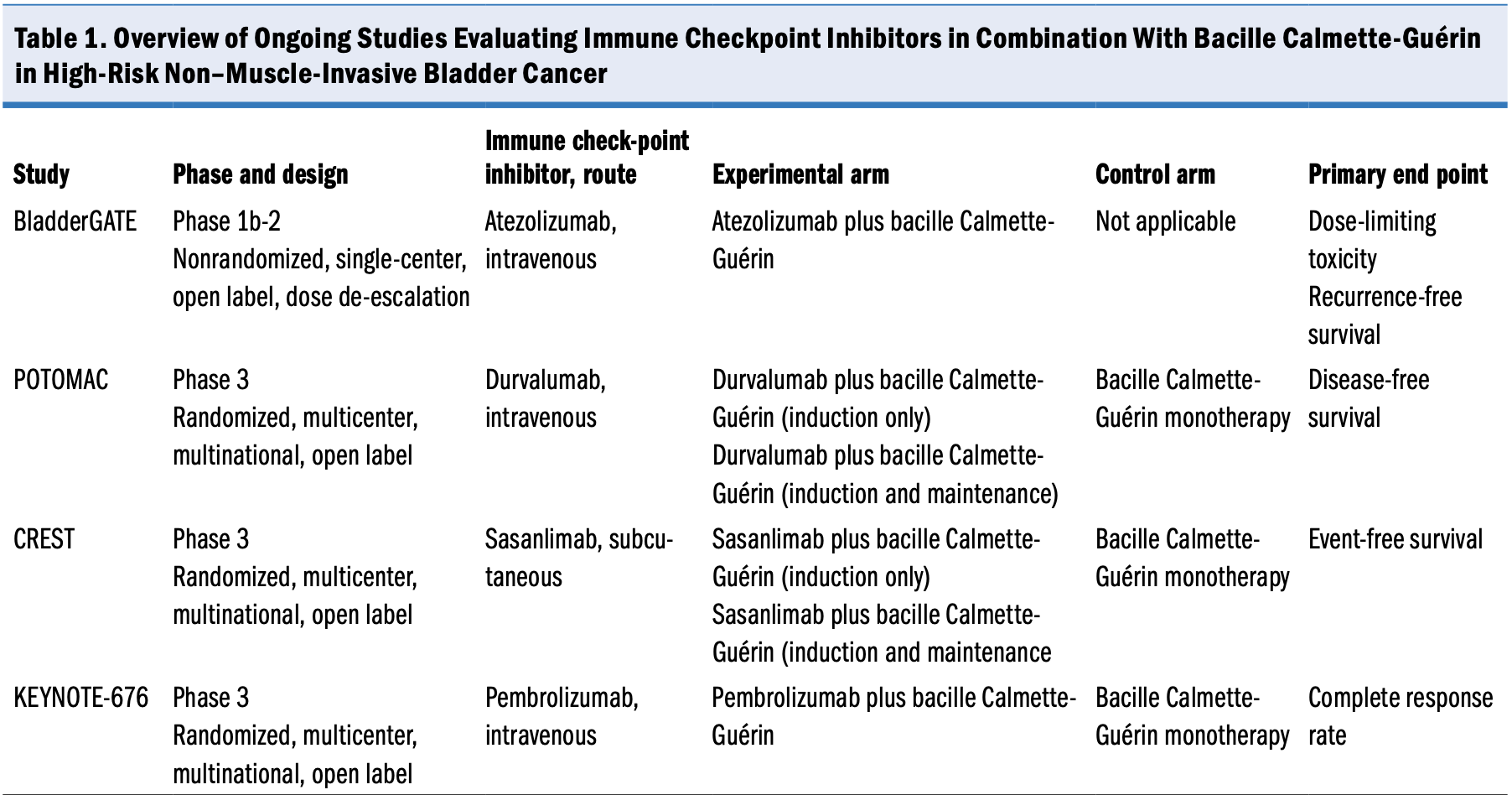
Several studies are underway to evaluate the safety and efficacy of immune checkpoint inhibitors in combination with bacille Calmette-Guérin vs the current standard of care (bacille Calmette-Guérin monotherapy) for patients with high-risk NMIBC that is either bacille Calmette-Guérin naive or recurrent after bacille Calmette-Guérin induction (Table 1). If the results of these studies are positive, then the standard-of-care treatment for patients with high-risk NMIBC may change substantially. This article summarizes 3 ongoing phase 3 studies and 1 early-phase study of immune checkpoint inhibitor therapy for NMIBC.
POTOMAC Study
Assessment of Efficacy and Safety of Durvalumab Plus BCG Compared to the Standard Therapy With BCG in Non-muscle Invasive Bladder Cancer (POTOMAC; ClinicalTrials.gov identifier NCT03528694) is a randomized, multicenter, multinational, open-label phase 3 trial evaluating the efficacy and safety of durvalumab in combination with bacille Calmette-Guérin vs current standard-of-care bacille Calmette-Guérin monotherapy for patients with bacille Calmette-Guérin–naive, high-risk NMIBC.9 Durvalumab is a selective, high-affinity, engineered human immunoglobulin G1 mAb that blocks PD-L1 from binding to PD-1 and CD80. The mAb has a manageable safety and tolerability profile. Inhibition of PD-L1 with durvalumab in combination with bacille Calmette-Guérin may improve the response rate and duration of tumor response.9
The primary end point for POTOMAC is disease-free survival. Secondary end points include the proportion of patients alive and disease free at 24 months, overall survival at 5 years, pharmacokinetics, immunogenicity, safety and tolerability, and health-related quality of life.
Approximately 1018 patients have been enrolled to date and randomly assigned (1:1:1) to 1 of the following arms:
- Experimental: durvalumab (1 year) plus bacille Calmette-Guérin (induction with maintenance period)
- Experimental: durvalumab (1 year) plus bacille Calmette-Guérin (induction only)
- Control (standard of care): bacille Calmette-Guérin monotherapy (induction and 2 years of maintenance)
Random assignment was stratified by high-risk papillary disease and carcinoma in situ (CIS). The first participant was enrolled in May 2018; primary completion is anticipated in fall 2024, with full study completion expected in September 2025.10
CREST Study
A Study of Sasanlimab in People With Non-muscle Invasive Bladder Cancer (CREST; ClinicalTrials.gov identifier NCT04165317) is a randomized, multicenter, multinational, open-label phase 3 study evaluating the efficacy and safety of sasanlimab in combination with bacille Calmette-Guérin vs current standard-of-care bacille Calmette-Guérin monotherapy for patients with bacille Calmette-Guérin–naive, high-risk NMIBC.11 Sasanlimab (PF-06801591) is a humanized immunoglobulin G4 mAb that selectively binds PD-1 and prevents the interaction between PD-1 and the PD-L1/PD-L2 expressed by cancer cells. The subcutaneous administration route and monthly dose of sasanlimab may offer enhanced convenience for patients and clinical practices while maintaining a safety and efficacy profile aligned to other PD-1/PD-L1 inhibitors administered intravenously.11
The primary end point of CREST is to demonstrate that sasanlimab in combination with bacille Calmette-Guérin (induction, with or without a maintenance period) is superior to bacille Calmette-Guérin monotherapy (induction and maintenance period) in prolonging event-free survival. Additional end points include overall survival, complete response, duration of complete response, time to recurrence of low-grade disease, time to cystectomy, disease-specific survival, safety, health-related quality of life, pharmacokinetics, and immunogenicity.
- Approximately 1070 participants were randomly assigned (1:1:1) to 1 of the following arms:
- Experimental: sasanlimab plus bacille Calmette-Guérin (induction with maintenance period)
- Experimental: sasanlimab plus bacille Calmette-Guérin (induction only)
- Control (standard of care): bacille Calmette-Guérin monotherapy for up to 25 cycles
Random assignment was stratified based on the presence of CIS and patient geography (United States vs western Europe and Canada vs the rest of the world).
The first participant was enrolled in December 2019. Primary completion is expected in December 2024, with study completion anticipated in winter 2026.12
KEYNOTE-676 Study
The Efficacy and Safety of Pembrolizumab in Combination With Bacillus Calmette-Guerin in High-Risk Non-Muscle Invasive Bladder Cancer (KEYNOTE-676) study (ClinicalTrials.gov identifier NCT03711032) is a randomized, multicenter, multinational, open-label phase 3 study to evaluate the efficacy and safety of pembrolizumab in combination with bacille Calmette-Guérin vs current standard-of-care bacille Calmette-Guérin monotherapy for patients with high-risk NMIBC that is either persistent or recurrent following bacille Calmette-Guérin induction or that is bacille Calmette-Guérin naive.13
Pembrolizumab is a humanized mAb that binds PD-1 with high affinity and selectivity, preventing PD-1 from interacting with its ligands, PD-L1 and PD-L2. Pembrolizumab provides effective and durable antitumor activity against multiple cancers and has been approved to treat a variety of cancer types.14 The effectiveness of pembrolizumab monotherapy in treating locally advanced and metastatic urothelial carcinoma has been demonstrated in several studies,14,15 including the phase 3 Study of Pembrolizumab Versus Paclitaxel, Docetaxel, or Vinflunine for Participants With Advanced Urothelial Cancer (KEYNOTE-045; ClinicalTrials.gov identifier NCT02256436),16 the phase 2 Study of Pembrolizumab in Participants With Advanced Urothelial Cancer (KEYNOTE-052; ClinicalTrials.gov identifier NCT02335424),17 and the phase 2 Study of Pembrolizumab and Pembrolizumab With Other Investigational Agents in Participants With High Risk Non-muscle Invasive Bladder Cancer (KEYNOTE-057; ClinicalTrials.gov identifier NCT02625961).18
The primary end point of KEYNOTE-676 is the complete
response rate in patients with CIS. Secondary end points are duration of response in patients with CIS, event-free survival, recurrence-free survival, time to cystectomy, overall survival, disease-specific survival, safety and tolerability, and patient-reported outcomes.
Approximately 1405 participants are anticipated to be randomly assigned (1:1) to 1 of the following arms:
- Experimental: pembrolizumab plus bacille Calmette-Guérin
- Control (standard of care): bacille Calmette-Guérin monotherapy
Bacille Calmette-Guérin induction therapy is followed by maintenance therapy (once weekly for 3 weeks at months 3, 6, 12, 18, 24, 30, and 36) in both arms. Treatment will continue for approximately 2 years (pembrolizumab) or 3 years (bacille Calmette-Guérin) or until confirmed persistent or recurrent high-risk NMIBC or disease progression to MIBC or metastatic bladder cancer, unacceptable toxicity, or withdrawal occurs. Random assignment to treatment will be stratified by PD-L1 combined positive score (≥10 or <10), histology (CIS or non-CIS), and NMIBC disease history (persistence or recurrence, 0 to ≤6 months; recurrence, >6 to ≤12 months; or recurrence, >12 to ≤24 months).
The first participant was enrolled in December 2018. Primary completion is anticipated in December 2025, with study completion expected in fall 2028.19
BladderGATE Study
Atezolizumab and BCG in High Risk BCG naïve Non-muscle Invasive Bladder Cancer Patients (BladderGATE; ClinicalTrials.gov identifier NCT04134000) is a nonrandomized, single-center, open-label, dose–de-escalation phase 1b-2 study to evaluate atezolizumab in combination with bacille Calmette-Guérin in patients with bacille Calmette-Guérin–naive, high-risk NMIBC.20 Atezolizumab is an immunoglobulin G1 monoclonal antibody (mAb) that targets PD-L1 and has previously demonstrated efficacy and tolerability in patients with metastatic urothelial cancer.20 Atezolizumab in combination with standard bacille Calmette-Guérin could provide benefits for patients with NMIBC.20,21
The primary outcome measures of the trial are dose-limiting toxicity and recurrence-free survival. Secondary outcome measures include incidence of treatment-emergent adverse events and a variety of patient-reported outcomes.
Approximately 40 participants have been enrolled to date. Participants receive 1200 mg atezolizumab intravenously on day 1 of each 21-day cycle (for a maximum of 52 weeks) plus bacille Calmette-Guérin weekly for 6 weeks (ie, induction period), followed by maintenance dosing weekly for 3 weeks at weeks 12, 24, and 48. Follow-up continues until recurrence of disease, symptomatic deterioration, intolerable toxicity, any medical condition that may jeopardize the patient’s safety, use of another nonprotocol anticancer therapy, or pregnancy occurs. The first participant was enrolled in February 2020; study completion and readout are anticipated in fall 2024.22
Interim results available from this study include data for 36 patients. Their median age was 70 years, 86% of the sample were men, and 44% of patients had a tumor size of at least 3 cm. With a median follow-up of 22 months, 56% of patients had completed bacille Calmette-Guérin treatment, and 89% had received adequate bacille Calmette-Guérin treatment (5 induction instillations plus at least 2 maintenance instillations). Thirteen patients (36%) discontinued atezolizumab because of immune-related adverse events, an early disease relapse, or progressive disease. All immune-related adverse events were resolved, and no deaths were reported. Preliminary 2-year disease-free survival is 72.8% (95% CI, 56.1%-89.5%). The interim results indicate that the combination strategy of atezolizumab plus bacille Calmette-Guérin up front in patients with high-risk NMIBC appears feasible, with a manageable safety profile.
Conclusion
Immune checkpoint inhibitors have transformed the field of cancer immunotherapy through their demonstrated outcomes of prolonged survival and maintained quality of life. The application of these inhibitors in NMIBC treatment therefore presents a promising way to address current clinical concerns of recurrence and progression rates, treatment intolerability, bacille Calmette-Guérin shortages and accessibility challenges, and the need for alternatives to radical cystectomy. The ongoing trials described in this review will help oncologists better understand immune checkpoint inhibitor risk-benefit profiles, particularly in terms of immune-related adverse events vs response and survival rates. Their results may substantially change the standard-of-care treatment for patients with high-risk NMIBC.
References
1. Passarelli R, Packiam VT. Contemporary treatment of NMIBC—is it time to move on from BCG? J Clin Med. 2024;13(14):4112. doi:10.3390/jcm13144112
2. Woldu SL, Bagrodia A, Lotan Y. Guideline of guidelines: non-muscle-invasive bladder cancer. BJU Int. 2017;119(3):371-380. doi:10.1111/bju.13760
3. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49. doi:10.3322/caac.21820
4. Chang SS, Boorjian SA, Chou R, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO Guideline. J Urol. 2016;196(4):1021-1029. doi:10.1016/j.juro.2016.06.049
5. Alturki NA. Review of the immune checkpoint inhibitors in the context of cancer treatment. J Clin Med. 2023;12(13):4301. doi:10.3390/jcm12134301
6. Hussaini S, Chehade R, Boldt RG, et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors—a systematic review and meta-analysis. Cancer Treat Rev. 2021;92:102134. doi:10.1016/j.ctrv.2020.102134
7. Audisio A, Buttigliero C, Delcuratolo MD, et al. New perspectives in the medical treatment of non-muscle-invasive bladder cancer: immune checkpoint inhibitors and beyond. Cells. 2022;11(3):357. doi:10.3390/cells11030357
8. de Jong FC, Rutten VC, Zuiverloon TCM, Theodorescu D. Improving anti-PD-1/PD-L1 therapy for localized bladder cancer. Int J Mol Sci. 2021;22(6):2800. doi:10.3390/ijms22062800
9. De Santis M, Abdrashitov R, Hegele A, et al. A phase III, randomized, open-label, multicenter, global study of durvalumab and bacillus Calmette-Guérin (BCG) versus BCG alone in high-risk, BCG-naïve non-muscle-invasive bladder cancer (NMIBC) patients (POTOMAC). J Clin Oncol. 2019;37(7_suppl): Abstract TPS500. doi:10.1200/JCO.2019.37.7_suppl.TPS50
10. Assessment of efficacy and safety of durvalumab plus BCG compared to the standard therapy with BCG in non-muscle invasive bladder cancer (POTOMAC). ClinicalTrials.gov. Updated August 28, 2024. Accessed September 3, 2024. https://clinicaltrials.gov/study/NCT03528694
11. Steinberg GD, Shore ND, Redorta JP, et al. CREST: phase III study of sasanlimab and Bacillus Calmette-Guérin for patients with bacillus Calmette-Guérin-naïve high-risk non-muscle-invasive bladder cancer. Future Oncol. 2024;20(14):891-901. doi:10.2217/fon-2023-0271
12. A study of sasanlimab in people with non-muscle invasive bladder cancer (CREST). ClinicalTrials.gov. Updated August 9, 2024. Accessed August 19, 2024. https://clinicaltrials.gov/study/NCT04165317
13. Shore ND, Nishiyama H, Shariat SF, et al. Phase 3 KEYNOTE-676 cohort A: bacillus Calmette-Guérin (BCG) with or without pembrolizumab for high-risk (HR) non–muscle invasive bladder cancer (NMIBC) that persists/recurs after BCG induction. J Clin Oncol. 2024;42(4_suppl): Abstract TPS722. doi:10.1200/JCO.2024.42.4_suppl.TPS72
14. Keytruda (pembrolizumab) injection. Package insert. Merck & Co, Inc; 2024. Accessed September 9, 2024. https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf
15. Kumat AM, Shore N, Hahn N, et al. KEYNOTE-676: phase III study of BCG and pembrolizumab for persistent/recurrent high-risk NMIBC. Future Oncol. 2020;16(10):507-516. doi:10.2217/fon-2019-0817
16. Bellmunt J, de Wit R, Vaughn DJ, et al; KEYNOTE-045 Investigators. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015-1026. doi:10.1056/NEJMoa163683
17. Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483-1492. doi:10.1016/S1470/2045(17)30616-2
18. de Wit R, Kulkarni GS, Uchio E, et al. Health-related quality of life (HRQoL) and updated follow-up from KEYNOTE-057: phase II study of pembrolizumab (pembro) for patients (pts) with high-risk (HR) non-muscle invasive bladder cancer (NMIBC) unresponsive to bacillus Calmette-Guérin (BCG). Ann Oncol. 2019;30(suppl 5):V364-V365.
19. Efficacy and safety of pembrolizumab (MK-3475) in combination with bacillus Calmette-Guerin (BCG) in high-risk non-muscle invasive bladder cancer (HR NMIBC) (MK-3475-676/KEYNOTE-676). ClinicalTrials.gov. Updated August 26, 2024. Accessed September 3, 2024. https://clinicaltrials.gov/study/NCT03711032
20. Castellano D, de Velasco G, Villarrubia JE, et al. BladderGATE: atezolizumab + intravesical BCG (bacillus Calmette-Guerin) upfront combination in patients with high risk non-muscle invasive bladder cancer (NMIBC)—phase I-II ONCOSUR study. J Clin Oncol. 2024;42(4_suppl): Abstract 595. doi:10.1200/JCO.2024.42.4_suppl.595
21. van der Heijden MS, Loriot Y, Durán I, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma: a long-term overall survival and safety update from the phase 3 IMvigor211 clinical trial. Eur Urol. 2021;80(1):7-11. doi:10.1016/j.eururo.2021.03.024
22. Atezolizumab and BCG in high risk BCG naïve non-muscle invasive bladder cancer (NMIBC) patients (BladderGATE). ClinicalTrials.gov. Updated May 21, 2024. Accessed August 19, 2024. https://clinicaltrials.gov/study/NCT04134000
Article Information
Published: September 13, 2024.
Conflict of Interest Disclosures: Dr Shore is an investigator in the POTOMAC, CREST, and KEYNOTE-676 trials and an advisor to Merck, Pfizer, and AstraZeneca.
Funding/Support: None.
Author Contributions: Dr Shore is solely responsible for the content of this article.
Data Availability Statement: All data presented in this article are available through the sources cited in the text.
Acknowledgments: Dr Shore would like to thank Janelle Arrambide for her assistance in preparing the content of this article.
Citation: Shore N. Immune checkpoint inhibitors for the treatment of non–muscle-invasive bladder cancer: an overview of ongoing clinical trials. Rev Urol. 2024;23(3):e77-e82.
Corresponding author: Neal Shore, MD, Medical Director, Carolina Urologic Research Center, 823 82nd Pkwy, Myrtle Beach, SC 29572 (nshore@gsuro.com)