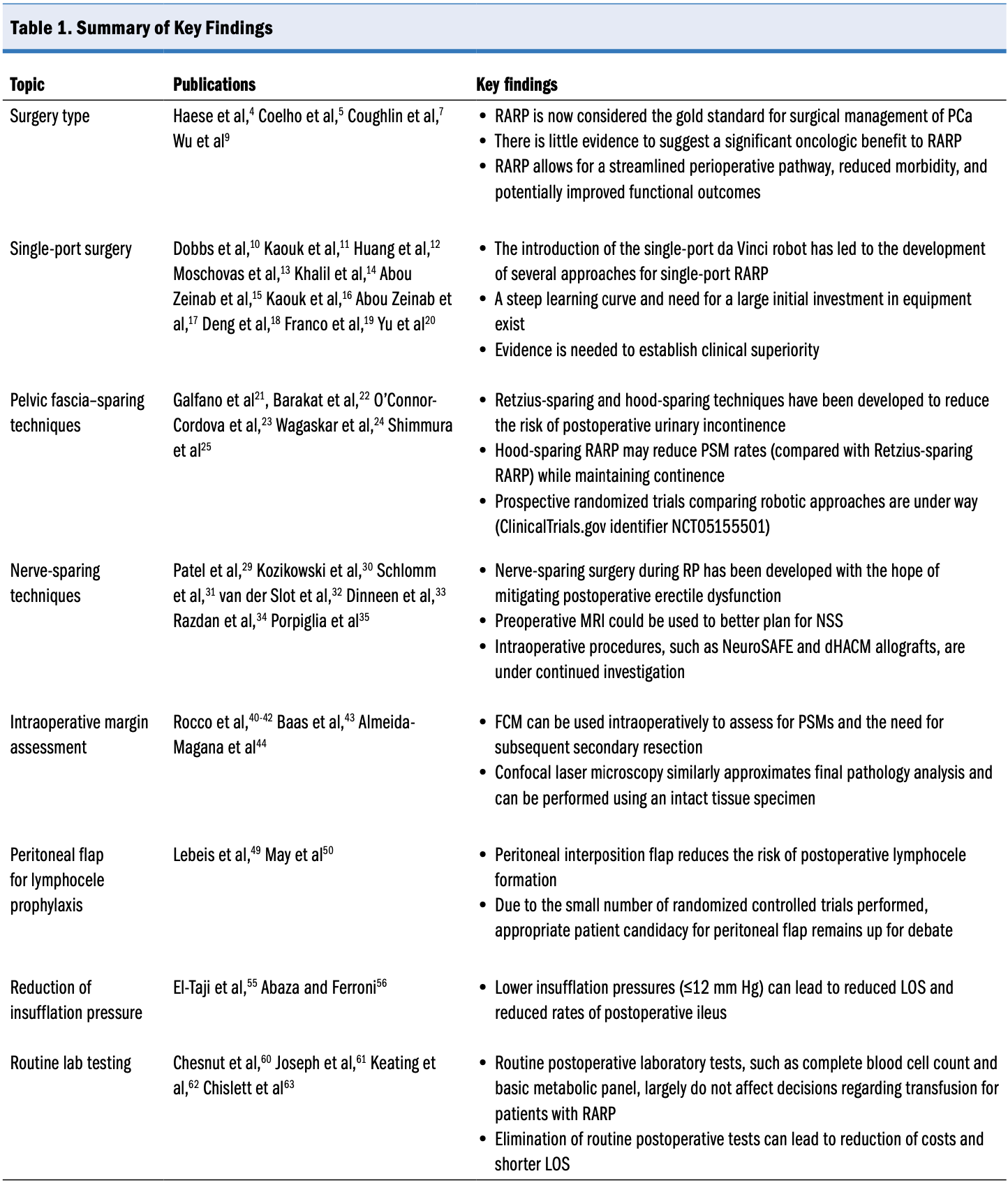
Introduction
Prostate cancer (PCa) is the second-most commonly diagnosed cancer and fifth-leading cause of death among men worldwide. It is estimated that 1 in 8 men will receive a diagnosis of PCa in their lifetime and that nearly 300 000 men in the United States will receive new diagnoses of PCa in 2024.1,2 Radical prostatectomy (RP) is the standard-of-care surgical treatment for patients with localized PCa and encompasses a range of techniques, including open, laparoscopic, and robot-assisted laparoscopic approaches. In the past several decades, the use of robot-assisted RP (RARP) has increased dramatically; it is estimated that more than 85% of RPs are currently performed using the Intuitive Surgical da Vinci platform.3,4 This review characterizes recent advances in RP, from clinical management to surgical technique, and directions for future research.
Surgical Technique
Surgery Type
Abbreviations
dHACM dehydrated human amnion/chorion membrane
FCM fluorescence confocal microscopy
LOS length of stay
MRI magnetic resonance imaging
NeuroSAFE intraoperative neurovascular structure- adjacent frozen-section examination
NSS nerve-sparing surgery
PCa prostate cancer
PSM positive surgical margin
RARP robotic-assisted radical prostatectomy
RP radical prostatectomy
SP single port
Radical prostatectomy has been performed for more than 100 years, with successful clinical outcomes reported across all types of surgery—open RP, laparoscopic RP, and RARP. In recent decades, RARP has begun to dominate the surgical landscape for PCa, with reduced recovery periods and substantially decreased need for blood transfusions.5,6 In high-volume centers, both open RP and RARP result in excellent oncologic and functional outcomes when performed by the same surgeons. Robot-assisted RP, however, is associated with modest improvement in surgical outcomes, including lower median intraoperative blood loss and transfusion rates.4 Shorter length of stay (LOS) has also been consistently demonstrated with RARP.7,8 In addition, a retrospective analysis demonstrated that compared with both and laparoscopic RP, RARP was associated with improvements in immediate postoperative outcomes as well as long-term functional outcomes.9 Given its superiority in surgical outcomes and similar to modest improvement in oncologic and functional outcomes, RARP has become the standard of care for PCa. As a result, we focus on novel developments in RARP that may offer further incremental improvements in surgical, oncologic, or functional outcomes that would be unattainable with an open approach.
Single-Port Surgery
Since 2018, the da Vinci SP system has been approved for urologic surgery, and its use has ushered in a new era of innovation for RARP surgery. The novel single-port (SP) system allows for the insertion of 3 articulating endoscopic instruments and a camera through a single laparoscopic trocar, facilitating transperitoneal, extraperitoneal, transvesical, and transperineal approaches to RARP.
Transperitoneal SP-RARP replicates the standard multiarm transperitoneal robotic approach.10,11 Comparative pooled analysis between transperitoneal SP-RARP and multiport RARP at high-volume centers found that perioperative and pathologic outcomes were nearly equivalent between the 2 cohorts.12 In a separate paired analysis, patients undergoing SP-RARP had longer operative times with less blood loss compared with patients undergoing multiport RARP, but no clinically substantial difference in postoperative pain scores was reported.13
The limitations of transperitoneal SP-RARP, including limited range of motion and lack of sweeping power, encouraged the development of alternative approaches to SP-RARP. In bypassing the peritoneal cavity, extraperitoneal SP-RARP limits peritoneal inflammation from insufflation and reduces bowel manipulation, but this technique is complicated in patients who have undergone prior procedures in the extraperitoneal space.14 Comparative analysis between extraperitoneal SP-RARP and transperitoneal SP-RARP demonstrated shorter LOS and increased rate of same-day discharge in patients undergoing extraperitoneal SP-RARP, despite longer total operative time and greater estimated blood loss. No difference was noted in overall intraoperative or postoperative complication rates or positive surgical margin (PSM) rates.15
The transvesical approach to SP-RARP also provides extraperitoneal access while avoiding potential adhesions from prior abdominal surgery, unnecessary dissection and mobilization of the bladder, bowel mobilization, and Trendelenburg positioning.16 Early reports from several authors have supported the safety and efficacy of this novel approach and highlighted the early return of continence.17 Most notably, a retrospective analysis from Deng et al18 reported that 90% of patients recovered continence immediately after catheter removal, with 100% continence at 3 months. Pelvic lymph node dissection, especially extended lymphadenectomy, may be limited with a transvesical approach, given that the procedure is performed via the bladder neck. Consequently, this technique is not recommended in patients with a high risk of lymph node metastasis.16
Transperineal SP-RARP is modeled after the original approach to RP from 1905; however, its application is limited to select cases in centers with requisite expertise because of its technical complexity and narrow operative space.19 Despite its feasibility, the steep learning curve associated with transperineal SP-RARP has limited its adoptability.20
Altogether, preliminary results across a variety of approaches demonstrate that SP-RARP is safe and feasible, offering outcomes comparable to standard multiport RARP. The learning curve and additional equipment required at this time, however, do not encourage a transition toward SP-RARP as a new standard of surgical care. Long-term, high-quality data are necessary to validate this approach’s clinical equivalency or superiority.
Pelvic Fascia–Sparing Techniques
Recently, novel surgical techniques have been developed that focus on preserving or restoring key pelvic anatomic structures to improve patient quality of life following RP. Most relevant are Retzius-sparing and hood-sparing techniques, and both are intended to reduce the risk of short-term and long-term urinary incontinence. Galfano et al21 introduced the Retzius-sparing technique in 2010, outlining a technique for RP that focused on sparing the retropubic space with the goal of preserving the natural pelvic anatomy.
Retzius-sparing RARP (RS-RARP) has been directly compared with conventional RARP across several small randomized controlled trials and prospective studies. A systematic review and meta-analysis from Barakat et al22 showed a clinically significant advantage for RS-RARP in terms of urinary continence recovery at 3 and 6 months compared with standard RARP. The authors also noted clinically significant increases in PSM rates, however, in tumors at pathologic stage pT2 or lower and pT3 or greater. No significant difference was noted in erectile function or overall postoperative complication rates.22 A meta-analysis by O’Connor-Cordova et al23 likewise identified greater continence recovery at 1 and 3 months following RS-RARP compared with conventional RARP. In contrast to the data from Barakat et al,22 however, O’Connor-Cordova et al23 found no difference in PSM rates, regardless of pathologic stage. Furthermore, no differences were noted in potency, estimated blood loss, LOS, operation time, or complication rates.23 Risk of increased incidence of PSM remains a concern with the Retzius-sparing technique, albeit in the context of clear improvement in continence recovery postoperatively. Additional quality studies focusing on PSM rates in RS-RARP are necessary to validate the safety of this approach with respect to oncologic outcomes.
Wagaskar et al24 subsequently developed the hood-sparing technique to address the potentially increased PSM rates found with the Retzius-sparing technique. The hood-sparing technique, which preserves the detrusor apron, endopelvic fascia, and puboprostatic ligaments in a “hood” appearance, is thought to provide additional support to the membranous urethra, external sphincter, and vesicourethral anastomosis, thereby preserving continence. In an initial series of 300 patients, continence rates at 1, 3, and 6 months following catheter removal were 83%, 91%, and 94%, respectively, with a PSM rate of 6%.24 In a similar retrospective analysis, however, Shimmura et al25 reported a PSM rate of 16%. The traditional anterior approach to RARP in the hood-sparing technique, which will be more familiar to surgeons, may offer an explanation for its improved PSM rates compared with the Retzius-sparing technique.24-26 Validation of these findings—in particular, the superiority of the hood-sparing technique in minimizing PSM rates while preserving early continence recovery—will require quality randomized controlled trials at high-volume, experienced tertiary centers.27
Abbreviations: dHACM, dehydrated human amnion/chorion membrane; FCM, fluorescence confocal microscopy; LOS, length of stay; MRI, magnetic resonance imaging; NeuroSAFE, intraoperative neurovascular structure-adjacent frozen-section examination; NSS, nerve-sparing surgery; PCa, prostate cancer; PSM, positive surgical margin; RARP, robotic-assisted radical prostatectomy; RP, radical prostatectomy
Nerve-Sparing Techniques
Nerve-sparing surgery (NSS) in RP, while designed to mitigate erectile dysfunction as a postoperative adverse effect, remains challenging. Several intraoperative techniques have been developed to protect or spare neural anatomy during RP to preserve sexual function. In general, optimal functional outcomes are achieved through athermal dissection of the neurovascular bundles bilaterally, without traction, along the correct planes.28 Direct comparison of nerve-sparing surgical techniques and determination of any superiority of one over another remain difficult because of the multifactorial nature of sexual potency and the challenges of postoperative assessment.
Several methodologies have been developed to balance NSS with the risk of increased PSM rates in cases in which PCa may extend beyond the capsule into the neurovascular bundles. The use of magnetic resonance imaging (MRI) before biopsy was analyzed by Patel et al29 for its impact on surgical outcomes; this approach demonstrated greater use of bilateral NSS and improved cancer control in terms of biochemical recurrence compared with a non-MRI approach, adjusting for known prognostic factors.29 A meta-analysis from Kozikowski et al30 demonstrated that modification of NSS based on MRI was frequent but had an uncertain impact on PSM rate. Additional prospective analysis is necessary to validate preoperative use of MRI as a prognostic factor for performing NSS.
Recent investigation has also focused on intra-operative neurovascular structure-adjacent frozen-section examination (NeuroSAFE), a novel technique, and its impact on the rate of NSS and oncologic outcomes. During the NeuroSAFE technique, intraoperative fresh-frozen section analysis of the posterolateral aspect of the prostatic margin is performed to assess whether cancer extends beyond the capsule. In the case of a positive result of pathologic testing, the ipsilateral neurovascular bundle is resected along with the rectolateral part of the Denonvilliers fascia.31 A validation study performed by van der Slot et al32 compared RARP with use of NeuroSAFE to standard RARP. NeuroSAFE was found to enable more frequent unilateral and bilateral NSS without negatively affecting PSM rates or biochemical recurrence.32 A single-blind, multicenter randomized controlled trial of NeuroSAFE is currently under way, with an expected completion date in 2025.33
The use of dehydrated human amnion/chorion membrane (dHACM) allografts placed on the neurovascular bundles during surgery has also been investigated to mitigate the minimal nerve injury that takes place during all NSS. Cytokines, growth factors, and neurotrophic factors in dHACM are thought to accelerate the healing process, promoting earlier return of erectile function. Binary logistic regression of a matched retrospective cohort of 1400 patients who underwent full bilateral nerve-sparing RARP demonstrated that dHACM allograft placement was an independent, statistically significant predictor of potency at 1 year.34 Similar efficacy in improvement of potency postoperatively has been reported in analysis of the application of chitosan membrane to the neurovascular bundle and of the application of a hyaluronic acid/carboxymethyl cellulose membrane to the prostatic bed and neurovascular plate.35,36 Because earlier promising measures have failed to demonstrate efficacy, randomized controlled trials are needed.37
Finally, hydrodissection has been investigated for its potential to preserve functional outcomes without increasing PSM rates. Pedraza et al38 described the saline-assisted fascial exposure technique, in which a low-pressure injection of saline solution into the periprostatic fascia achieves an atraumatic dissection of the neural hammock, and its impact on erectile function, urinary continence, and oncologic outcomes following RARP. In patients who received RARP with saline-assisted fascial exposure compared with RARP alone, better Sexual Health Inventory for Men scores were reported at 6, 13, 26, and 52 weeks after surgery. Baseline Sexual Health Inventory for Men score and use of the saline-assisted fascial exposure technique were independent predictors of erectile function recovery in this study, which requires multicenter validation to confirm efficacy.38
Sexual potency is a major quality-of-life concern among patients before RP, and addressing potency through the methods discussed here is part of the “pentafecta” of outcomes that patients will soon come to expect as functional efficacy improves.6 The techniques and technologies described earlier outline a new standard approach to maximizing potency in patients following RP, from preoperative MRI to guide surgical decision-making to the use of intraoperative NeuroSAFE or similar technology to understand exact patient pathology to the grafting of neurotrophic factor–containing biomaterials that promote postoperative healing. Modifications in addition to surgical technique, such as clipless, lateral pedicle control and pelvic lymph node dissection in RARP with bipolar energy (vs the standard RARP technique with clips), may shorten operative time, limit clip erosion, and prevent perianastomotic stone formation. Despite concerns for increased risk of nerve injury secondary to bipolar energy use for prostatic pedicle dissection, recent studies have demonstrated similar oncologic and functional outcomes without increased risk of complications.39 Individually or together, these efforts have the potential to reduce erectile dysfunction following RP, although they require further assessment in broader randomized studies.
Intraoperative Margin Assessment
Reports of increased PSM rates following nerve-sparing techniques during RP have resulted in efforts to use fluorescence confocal microscopy (FCM) to detect residual prostate tissue intraoperatively in the periprostatic environment. Compared with the previously described NeuroSAFE technique, FCM digital images can be acquired within 1 to 2 minutes per sample, permitting immediate microscopic reading by a pathologist outside the institution. In contrast, NeuroSAFE requires more than 30 minutes to perform in a fully equipped laboratory with an on-site pathologist.40 A group led by Rocco et al40-42 has published 3 studies on this topic, all of which found excellent agreement between FCM and subsequent hematoxylin-eosin pathology analysis. Their most recent study best elucidated the clinical application of FCM in PSM assessment, with 24 tissue specimens analyzed and subsequent secondary resection performed in 4 patients with PSMs detected intraoperatively. All 24 patients had negative surgical margins at the site adjacent to the neurovascular bundles after primary and, where applicable, secondary resection.42
Separately, Baas et al43 directly compared the performance of the Histolog Scanner (SamanTree Medical), a commercially available, portable laser confocal microscope, with the NeuroSAFE technique. Confocal laser microscopy performed by the Histolog Scanner had a calculated sensitivity of 86% and a specificity of 96% compared with the final pathologic analysis as well as substantial agreement with NeuroSAFE. In addition, the median procedure time for confocal laser microscopy was shorter than for NeuroSAFE, with statistical significance.43 The primary limitation of this technique, however, is that the surgeon or pathologist must cut the tissue specimen being analyzed, which necessitates expertise in sample processing to avoid inaccurate pathology results. Almeida-Magana et al44 address this limitation with the LaserSAFE technique, using the Histolog Scanner to process the entire posterolateral surface of intact RARP specimens en face. Across 31 RARP specimens, the sensitivity and specificity of LaserSAFE for diagnosis of PSM were 87.5% and 98.1%, respectively, with almost perfect agreement between LaserSAFE and final pathology analysis.44 Widespread, expedient, real-time analysis of PSMs during RP appears to be on the horizon with the advent of these novel techniques.
Peritoneal Flap for Lymphocele Prophylaxis
Although pelvic lymph node dissection has been shown to improve oncologic outcomes for patients with appropriate risk classification, the procedure is associated with complications. Symptomatic lymphocele occurs in roughly 2% to 10% of patients following RARP with pelvic lymph node dissection.45,46 Although many lymphoceles can resolve without intervention, some require drainage and can lead to patient discomfort.47,48 In 2015, Lebeis et al49 proposed a novel technique in which a peritoneal interposition flap is placed between the lateral wall of the bladder and the lymphadenectomy bed to prevent the formation of lymphoceles. Lymphoceles did not develop in any of the patients who received the peritoneal flap, but lymphoceles did form in 9 of 77 (11.7%) patients who did not receive the flap.49
Since the initial publication of the study by Lebeis et al,49 several groups have evaluated the technique they described. In 2024, May et al50 published a systematic review based on the results of 4 randomized controlled trials. Rates of symptomatic lymphocele were 3.6% (21/580) and 7.8% (46/588) in the peritoneal interposition flap and control groups, respectively, with a pooled odds ratio of 0.43.50 Although additional randomized controlled trials may be helpful to elucidate more details on technique and selection, peritoneal interposition flap placement remains a promising avenue for the reduction of lymphocele formation.
Reduction of Insufflation Pressure
Reduction of pneumoperitoneum insufflation pressure during surgery is another area of research that has coincided with the increased prevalence of minimally invasive RP. Laparoscopic RP has traditionally been performed at a standard operating pressure of 15 mm Hg, in line with other laparoscopic procedures.51 This technique has physiologic consequences, however, including reduced cardiac output, increased peak airway pressure, and metabolic acidosis.52-54 In an effort to reduce the associated burden on patients, there has been interest in exploring ways to reduce intraoperative pneumoperitoneum pressure.
A meta-analysis from El-Taji et al55 sought to understand the clinical impact of this technique and provide a consensus recommendation. Lower pressures during RARP (≤12 mm Hg) resulted in a statistically reduced LOS and rate of postoperative ileus. There were no differences in operative time, estimated blood loss, PSM, or complication rates.55 Furthermore, a randomized controlled trial by Abaza and Ferroni56 established statistically significant reductions in immediate, maximum, shoulder, and groin pain scores in patients with ultralow insufflation pressure with RARP (6 mm Hg) compared with standard insufflation pressure (15 mm Hg). Whereas there was no statistical difference in morphine equivalents intraoperatively or postoperatively, earlier flatulence was observed in the group with ultralow pressure.56 Although there is promising evidence supporting the use of lower pressures, further validation is necessary to establish a safe minimum for pneumoperitoneum insufflation pressure.
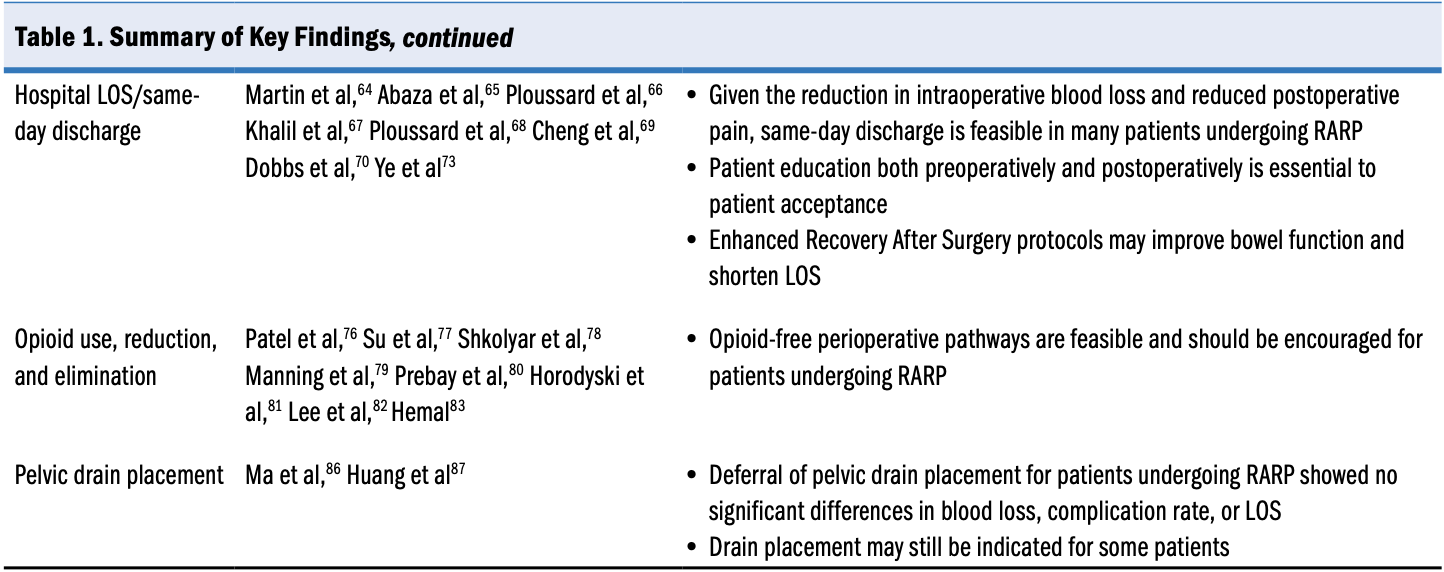
Perioperative Management
Routine Lab Testing
Routine postoperative tests, including complete blood cell count and basic metabolic panel, are frequently obtained following RP. Traditionally, these tests were performed to assess bleeding and the need for transfusion following open prostatectomy, but the increasing prevalence of minimally invasive surgical techniques has reduced the risk of significant bleeding and transfusion following RP to 0% to 3%.4,57-59 Clinical benefit and costs to the patient and hospital for routine postoperative tests should also be considered.
Recently, several studies have scrutinized the clinical utility of perioperative labs. Chesnut et al60 retrospectively analyzed a cohort of 3631 patients to determine the utility of 4-hour and 14-hour postoperative hemoglobin assessments in predicting the need for transfusion. No clinical decisions were made using information gleaned from the 4-hour assessment. In addition, only 44 patients (1.2%) received a blood transfusion, and 18 (41%) of these transfusions were based solely on laboratory test values. The financial burden of the unnecessary 4-hour test for the institution was $37 000.60 Joseph et al61 performed a similar analysis with a cohort of 3405 patients and found that of the 1.7% of patients who received a transfusion, 96% had clinical symptoms of anemia that would have prompted further testing.
Keating et al62 retrospectively reviewed 200 patients who underwent RARP and found that only 15 (7.5%) had laboratory test abnormalities that resulted in medical intervention, all of which were associated with prolonged inpatient stay. Most recently, Chislett et al63 retrospectively analyzed a 300-patient cohort and found that eliminating routine postoperative tests did not change the course of care and led to a median LOS that was 1 day shorter than the LOS for patients undergoing these tests, representing a substantial cost benefit for patients and the health care system. Based on the literature, there is reason to question the utility of routine postoperative tests following RARP because these tests may be an artifact of open procedures, which are associated with greater blood loss.
Hospital LOS/Same-Day Discharge
Decreased intraoperative blood loss and reduced perioperative pain with RARP raise the question of whether an inpatient stay is actually needed. Same-day discharge following RARP was first reported by Martin et al64 in 2010 in a cohort of 11 patients, all of whom were discharged without complication. Several studies have since corroborated that same-day discharge does not increase the risk of 30-day readmission compared with next-day discharge.65-67
Despite its reported safety, an overnight stay following RARP is still the standard of care at most institutions. In a countrywide retrospective analysis of all RARPs performed in France within 1 year, Ploussard et al68 found that only 184 of 9651 RARPs (1.9%) involved same-day discharge.68 The reduction in LOS also affects cost to patients and the hospital system. Abaza et al65 approximated a reduction in charges of $345 876 per year as a result of same-day discharge, with no increased cost resulting from emergency department visits or hospital readmissions. Cheng et al69 performed a time-driven, activity-based cost analysis and found a 19% reduction in cost to patients who had same-day discharge, with similar median satisfaction survey scores. Given the large volume of RARPs performed in the United States and worldwide each year, the potential fiscal impact of implementing same-day discharge is substantial.
Patient acceptance, in addition to historical precedence, could be a barrier to same-day discharge. Dobbs et al70 studied barriers to same-day discharge and found that postoperative pain, catheter discomfort, and insufficient education on pain management and catheter care were the primary factors inhibiting early discharge. Abaza et al65 emphasized the importance of proper education at consultation, preoperatively, and postoperatively to facilitate smooth discharge. These findings indicate that with proper education before surgery, patient acceptance is less a barrier than has previously been suggested.
In addition, several studies have examined the use of Enhanced Recovery After Surgery protocols. These protocols were first described by Kehlet71 in 1997 and have since been adopted in numerous specialties; the protocols include a variety of surgical, anesthesia, and nursing interventions that take place preoperatively, intraoperatively, and postoperatively.72 A 2020 meta-analysis of 10 studies found that the use of Enhanced Recovery After Surgery protocols following RP was associated with shorter time to first defecation, shorter time to first anal exhaust, shorter hospital LOS, and less nausea.73
Opioid Use, Reduction, and Elimination
In recent decades, opioid-related deaths have become an urgent public health issue in the United States. In 2021, 1 of every 22 deaths was attributed to unintentional opioid toxicity.74 An estimated 6% to 7% of patients experience persistent opioid use after surgical procedures, which evokes the need for conservative prescribing whenever possible.75 Overprescribing of opioids postoperatively is common and can lead to issues with medication disposal and diversion after the patient leaves the hospital.70
One of the earliest interventions aimed at reducing postoperative opioid use following RP was the Open, Laparoscopic, and Endoscopic Surgery Initiative in 2020. In this study, Patel et al76 developed a pre-post interventional trial aimed at reducing total oral morphine equivalents following RP. In the postinterventional arm, there was a 46.4% reduction in opioid prescriptions, a 26.5% reduction in opioid use, and a 13.5% increase in opioid disposal compared with the preinterventional arm.76 Additional analysis of this patient cohort demonstrated that inpatient opioid use, patient-reported pain scores, prior opioid use, and body mass index were all predictors of opioid use following discharge.77
A study by Shkolyar et al78 found that patients undergoing RARP were 35% less likely to experience new, persistent opioid use than patients undergoing open RP. Manning et al79 developed a multidisciplinary opioid-reduction pathway for patients undergoing RARP that used a series of educational, nursing, and nonopioid analgesic interventions. A statistically significant decrease in intraoperative and postoperative opioid use was noted for patients who underwent the intervention, underscoring the potential for widescale opioid de-escalation.79 These findings suggest that individualized opioid prescribing, combined with patient education on proper opioid use, may help reach a balance between undertreatment and overprescribing.
In addition to opioid reduction efforts, several studies have evaluated complete elimination of opioids in addition to opioid-reduction efforts following RP. Prebay et al80 and Horodyski et al81 developed opioid-free interventions to evaluate postoperative pain and found noninferior pain scores. Although opioid use will likely remain necessary for some patients, these findings suggest that it can be spared in the majority of patients. Lee et al82 performed a randomized noninferiority trial to compare the efficacy of a nonopioid multimodal analgesia protocol against standard opioid protocols. No significant differences in 24-hour postoperative pain scores were observed, and the nonopioid multimodal analgesia group exhibited a quicker return to normal bowel function than did the opioid group.82
An ongoing phase 2/3 trial is investigating the use of an opioid-free pathway following RARP.83 Patients randomly assigned to the experimental arm receive perioperative ketamine, ketorolac, and intravenous acetaminophen followed by postoperative ketorolac and oral acetaminophen; results are expected in 2025. Results of this trial will add power to previous findings regarding opioid-sparing RARP.
Pelvic Drain Placement
Pelvic drain placement following RP is a common practice to remove lymph, blood, urine, and other fluids from the patient. Notably, patients are more likely to experience pain and infection at the site of drain placement.84 Several studies have analyzed the need for pelvic drain placement after RARP. Abaza et al85 retrospectively reviewed more than 4600 interventions performed by a single surgeon, 3692 of which were prostatectomies. They noted that 99.6% of the patients undergoing RP did not receive a pelvic drain. In a 90-day follow-up, only 3 patients (0.08%) experienced urine leaks, which indicates that routine drain placement is unnecessary in the vast majority of patients. A 2023 meta-analysis of 6 studies comprising 1480 patients evaluated outcomes of RARP with drain placement vs RARP without drain placement.86 No significant differences in blood loss, complication rates, or hospital LOS were observed between the 2 groups. Huang et al87 specifically included opioid use following surgery as an end point and found no difference in complication rates or opioid use following surgery. Although certain circumstances may warrant drain placement, including extensive pelvic lymph node dissection or an anastomotic leak, findings suggest that drain placement can be deferred with appropriate clinical judgment in the majority of patients following RARP.84,88
Summary and Future Directions
As treatment for PCa continues to evolve, the goal of surgical intervention remains oncologic control while preserving functional outcomes and minimizing perioperative morbidity. Robotic prostatectomy currently allows for a streamlined perioperative pathway that can omit opioid use, routine laboratory tests, and pelvic drain placement and allow for same-day discharge. Techniques that may improve functional outcomes without compromising oncologic control have been developed (ie, anterior fascial or Retzius sparing), and these methods are being validated for potential superiority in prospective randomized studies. Novel emerging robotic platforms represent the most immediate advancement in the field. Robotic surgical systems with distinct functionality may eventually prove pivotal in the development of surgical techniques previously not deemed feasible using the da Vinci platform. Also importantly, these systems may improve surgeon ergonomics and reduce operative times. Reliability and efficacy in surgical and functional outcomes have been proven with the Hugo RAS system (Medtronic), as demonstrated in a comparative study between patients who underwent RARP with the da Vinci surgical system and patients who received RARP using the Hugo RAS system.89 The Revo-i surgical robotic system (Meere Company) and the Versius surgical robot (CMR Surgical) have also shown promise in RARP, albeit in limited patient cohorts.90-93
References
1. Wang L, Lu B, He M, Wang Y, Wang Z, Du L. Prostate cancer incidence and mortality: global status and temporal trends in 89 countries from 2000 to 2019. Front Public Health. 2022;10:811044. doi:10.3389/fpubh.2022.811044
2. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49. doi:10.3322/caac.21820
3. Iadeluca L, Mardekian J, Chander P, Hopps M, Makinson GT. The burden of selected cancers in the US: health behaviors and health care resource utilization. Cancer Manag Res. 2017;9:721-730. doi:10.2147/CMAR.S143148
4. Haese A, Knipper S, Isbarn H, et al. A comparative study of robot‐assisted and open radical prostatectomy in 10 790 men treated by highly trained surgeons for both procedures. BJU Int. 2019;123(6):1031-1040. doi:10.1111/bju.14760
5. Coelho RF, Rocco B, Patel MB, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a critical review of outcomes reported by high-volume centers. J Endourol. 2010;24(12):2003-2015. doi:10.1089/end.2010.0295
6. Patel VR, Sivaraman A, Coelho RF, et al. Pentafecta: a new concept for reporting outcomes of robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2011;59(5):702-707. doi:10.1016/j.eururo.2011.01.032
7. Coughlin GD, Yaxley JW, Chambers SK, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: 24-month outcomes from a randomised controlled study. Lancet Oncol. 2018;19(8):1051-1060. doi:10.1016/S1470-2045(18)30357-7
8. Gandaglia G, Sammon JD, Chang SL, et al. Comparative effectiveness of robot-assisted and open radical prostatectomy in the postdissemination era. J Clin Oncol. 2014;32(14):1419-1426. doi:10.1200/JCO.2013.53.5096
9. Wu SY, Chang CL, Chen CI, Huang CC. Comparison of acute and chronic surgical complications following robot-assisted, laparoscopic, and traditional open radical prostatectomy among men in Taiwan. JAMA Netw Open. 2021;4(8):e2120156. doi:10.1001/jamanetworkopen.2021.20156
10. Dobbs RW, Halgrimson WR, Madueke I, Vigneswaran HT, Wilson JO, Crivellaro S. Single-port robot-assisted laparoscopic radical prostatectomy: initial experience and technique with the da Vinci® SP platform. BJU Int. 2019;124(6):1022-1027. doi:10.1111/bju.14864
11. Kaouk J, Bertolo R, Eltemamy M, Garisto J. Single-port robot-assisted radical prostatectomy: first clinical experience using the SP surgical system. Urology. 2019;124:309. doi:10.1016/j.urology.2018.10.025
12. Huang MM, Patel HD, Wainger JJ, et al. Comparison of perioperative and pathologic outcomes between single-port and standard robot-assisted radical prostatectomy: an analysis of a high-volume center and the pooled world experience. Urology. 2021;147:223-229. doi:10.1016/j.urology.2020.08.046
13. Moschovas MC, Loy D, Patel E, Sandri M, Moser D, Patel V. Comparison between intra- and postoperative outcomes of the da Vinci SP and da Vinci Xi robotic platforms in patients undergoing radical prostatectomy. J Robot Surg. 2023;17(4):1341-1347. doi:10.1007/s11701-023-01563-5
14. Khalil MI, Joseph JV. Extraperitoneal single-port robot-assisted radical prostatectomy. J Endourol. 2021;35(S2):S100-S105. doi:10.1089/end.2021.0440
15. Abou Zeinab M, Beksac AT, Ferguson E, et al. Single-port extraperitoneal and transperitoneal radical prostatectomy: a multi-institutional propensity-score matched study. Urology. 2023;171:140-145. doi:10.1016/j.urology.2022.10.001
16. Kaouk J, Beksac AT, Abou Zeinab M, Duncan A, Schwen ZR, Eltemamy M. Single port transvesical robotic radical prostatectomy: initial clinical experience and description of technique. Urology. 2021;155:130-137. doi:10.1016/j.urology.2021.05.022
17. Abou Zeinab M, Beksac AT, Ferguson E, Kaviani A, Kaouk J. Transvesical versus extraperitoneal single-port robotic radical prostatectomy: a matched-pair analysis. World J Urol. 2022;40(8):2001-2008. doi:10.1007/s00345-022-04056-6
18. Deng W, Jiang H, Liu X, et al. Transvesical Retzius-sparing versus standard robot-assisted radical prostatectomy: a retrospective propensity score-adjusted analysis. Front Oncol. 2021;11:687010. doi:10.3389/fonc.2021.687010
19. Franco A, Pellegrino AA, De Nunzio C, et al. Single-port robot-assisted radical prostatectomy: where do we stand? Curr Oncol. 2023;30(4):4301-4310. doi:10.3390/curroncol30040328
20. Yu C, Xu L, Ye L, et al. Single-port robot-assisted perineal radical prostatectomy with the da Vinci XI system: initial experience and learning curve using the cumulative sum method. World J Surg Oncol. 2023;21(1):46. doi:10.1186/s12957-023-02927-9
21. Galfano A, Ascione A, Grimaldi S, Petralia G, Strada E, Bocciardi AM. A new anatomic approach for robot-assisted laparoscopic prostatectomy: a feasibility study for completely intrafascial surgery. Eur Urol. 2010;58(3):457-461. doi:10.1016/j.eururo.2010.06.008
22. Barakat B, Othman H, Gauger U, Wolff I, Hadaschik B, Rehme C. Retzius sparing radical prostatectomy versus robot-assisted radical prostatectomy: which technique is more beneficial for prostate cancer patients (MASTER Study)? A systematic review and meta-analysis. Eur Urol Focus. 2022;8(4):1060-1071. doi:10.1016/j.euf.2021.08.003
23. O’Connor-Cordova MA, Macias AGO, Sancen-Herrera JP, et al. Surgical and functional outcomes of Retzius-sparing robotic-assisted radical prostatectomy versus conventional robotic-assisted radical prostatectomy in patients with biopsy-confirmed prostate cancer. Are outcomes worth it? Systematic review and meta-analysis. Prostate. 2023;83(15):1395-1414. doi:10.1002/pros.24604
24. Wagaskar VG, Mittal A, Sobotka S, et al. Hood technique for robotic radical prostatectomy-preserving periurethral anatomical structures in the space of Retzius and sparing the pouch of Douglas, enabling early return of continence without compromising surgical margin rates. Eur Urol. 2021;80(2):213-221. doi:10.1016/j.eururo.2020.09.044
25. Shimmura H, Banno T, Nakamura K, et al. A single-center retrospective comparative analysis of urinary continence in robotic prostatectomy with a combination of umbilical ligament preservation and hood technique. Int J Urol. 2023;30(10):889-895. doi:10.1111/iju.15227
26. Galfano A, Secco S, Dell’Oglio P, et al. Retzius-sparing robot-assisted radical prostatectomy: early learning curve experience in three continents. BJU Int. 2021;127(4):412-417. doi:10.1111/bju.15196
27. Stangl-Kremser J, Kowalczyk K, Schaeffer EM, et al. Study protocol for a prospective, multi-centered randomized controlled trial comparing pelvic fascia-sparing radical prostatectomy with conventional robotic-assisted prostatectomy: the PARTIAL trial. Contemp Clin Trials. 2023;128:107168. doi:10.1016/j.cct.2023.107168
28. Kyriazis I, Spinos T, Tsaturyan A, Kallidonis P, Stolzenburg JU, Liatsikos E. Different nerve-sparing techniques during radical prostatectomy and their impact on functional outcomes. Cancers (Basel). 2022;14(7):1601. doi:10.3390/cancers14071601
29. Patel HD, Okabe Y, Rac G, et al. MRI versus non-MRI diagnostic pathways before radical prostatectomy: impact on nerve-sparing, positive surgical margins, and biochemical recurrence. Urol Oncol. 2023;41(2):104 e19-104 e27. doi:10.1016/j.urolonc.2022.10.012
30. Kozikowski M, Malewski W, Michalak W, Dobruch J. Clinical utility of MRI in the decision-making process before radical prostatectomy: systematic review and meta-analysis. PLoS One. 2019;14(1):e0210194. doi:10.1371/journal.pone.0210194
31. Schlomm T, Tennstedt P, Huxhold C, et al. Neurovascular structure-adjacent frozen-section examination (NeuroSAFE) increases nerve-sparing frequency and reduces positive surgical margins in open and robot-assisted laparoscopic radical prostatectomy: experience after 11,069 consecutive patients. Eur Urol. 2012;62(2):333-340. doi:10.1016/j.eururo.2012.04.057
32. van der Slot MA, den Bakker MA, Tan TSC, et al. NeuroSAFE in radical prostatectomy increases the rate of nerve-sparing surgery without affecting oncological outcome. BJU Int. 2022;130(5):628-636. doi:10.1111/bju.15771
33. Dinneen E, Grierson J, Almeida-Magana R, et al. NeuroSAFE PROOF: study protocol for a single-blinded, IDEAL stage 3, multi-centre, randomised controlled trial of NeuroSAFE robotic-assisted radical prostatectomy versus standard robotic-assisted radical prostatectomy in men with localized prostate cancer. Trials. 2022;23(1):584. doi:10.1186/s13063-022-06421-7
34. Razdan S, Bajpai RR, Razdan S, Sanchez MA. A matched and controlled longitudinal cohort study of dehydrated human amniotic membrane allograft sheet used as a wraparound nerve bundles in robotic-assisted laparoscopic radical prostatectomy: a puissant adjunct for enhanced potency outcomes. J Robot Surg. 2019;13(3):475-481. doi:10.1007/s11701-018-0873-7
35. Porpiglia F, Manfredi M, Checcucci E, et al. Use of chitosan membranes after nerve-sparing radical prostatectomy improves early recovery of sexual potency: results of a comparative study. BJU Int. 2019;123(3):465-473. doi:10.1111/bju.14583
36. Hinata N, Bando Y, Chiba K, et al. Application of hyaluronic acid/carboxymethyl cellulose membrane for early continence after nerve-sparing robot-assisted radical prostatectomy. BMC Urol. 2019;19(1):25. doi:10.1186/s12894-019-0458-4
37. Patel HD, Schwen ZR, Campbell JD, et al. Effect of erythropoietin on erectile function after radical prostatectomy: the ERECT randomized clinical trial. J Urol. 2021;205(6):1681-1688. doi:10.1097/JU.0000000000001586
38. Pedraza AM, Gupta R, Joshi H, et al. Saline-assisted fascial exposure (SAFE) technique to improve nerve-sparing in robot-assisted laparoscopic radical prostatectomy. BJU Int. 2024;133(4):451-459. doi:10.1111/bju.16238
39. Basourakos SP, Zhu A, Lewicki PJ, et al. Clipless robotic-assisted radical prostatectomy and impact on outcomes. Eur Urol Focus. 2022;8(5):1176-1185. doi:10.1016/j.euf.2021.06.010
40. Rocco B, Sighinolfi MC, Bertoni L, et al. Real-time assessment of surgical margins during radical prostatectomy: a novel approach that uses fluorescence confocal microscopy for the evaluation of peri-prostatic soft tissue. BJU Int. 2020;125(4):487-489. doi:10.1111/bju.15000
41. Rocco B, Sighinolfi MC, Cimadamore A, et al. Digital frozen section of the prostate surface during radical prostatectomy: a novel approach to evaluate surgical margins. BJU Int. 2020;126(3):336-338. doi:10.1111/bju.15108
42. Rocco B, Sarchi L, Assumma S, et al. Digital frozen sections with fluorescence confocal microscopy during robot-assisted radical prostatectomy: surgical technique. Eur Urol. 2021;80(6):724-729. doi:10.1016/j.eururo.2021.03.021
43. Baas DJH, Vreuls W, Sedelaar JPM, et al. Confocal laser microscopy for assessment of surgical margins during radical prostatectomy. BJU Int. 2023;132(1):40-46. doi:10.1111/bju.15938
44. Almeida-Magana R, Au M, Al-Hammouri T, et al. Improving fluorescence confocal microscopy for margin assessment during robot-assisted radical prostatectomy: the LaserSAFE technique. BJU Int. 2024;133(6):677-679. doi:10.1111/bju.16239
45. Feicke A, Baumgartner M, Talimi S, et al. Robotic-assisted laparoscopic extended pelvic lymph node dissection for prostate cancer: surgical technique and experience with the first 99 cases. Eur Urol. 2009;55(4):876-883. doi:10.1016/j.eururo.2008.12.006
46. Musch M, Klevecka V, Roggenbuck U, Kroepfl D. Complications of pelvic lymphadenectomy in 1,380 patients undergoing radical retropubic prostatectomy between 1993 and 2006. J Urol. 2008;179(3):923-929, discussion 928-929. doi:10.1016/j.juro.2007.10.072
47. Freid RM, Siegel D, Smith AD, Weiss GH. Lymphoceles after laparoscopic pelvic node dissection. Urology. 1998;51(5A suppl):131-134. doi:10.1016/s0090-4295(98)00074-0
48. Orvieto MA, Coelho RF, Chauhan S, Palmer KJ, Rocco B, Patel VR. Incidence of lymphoceles after robot‐assisted pelvic lymph node dissection. BJU Int. 2011;108(7):1185-1190. doi:10.1111/j.1464-410X.2011.10094.x
49. Lebeis C, Canes D, Sorcini A, Moinzadeh A. Novel technique prevents lymphoceles after transperitoneal robotic-assisted pelvic lymph node dissection: peritoneal flap interposition. Urology. 2015;85(6):1505-1509. doi:10.1016/j.urology.2015.02.034
50. May M, Gilfrich C, Bründl J, et al. Impact of peritoneal interposition flap on patients undergoing robot-assisted radical prostatectomy and pelvic lymph node dissection: a systematic review and meta-analysis of randomized controlled trials. Eur Urol Focus. 2024;10(1):80-89. doi:10.1016/j.euf.2023.07.007
51. Abbou CC, Hoznek A, Salomon L, et al. Laparoscopic radical prostatectomy with a remote controlled robot. J Urol. 2001;165(6 pt 1):1964-1966. doi:10.1097/00005392-200106000-00027
52. Taura P, Lopez A, Lacy AM, et al. Prolonged pneumoperitoneum at 15 mmHg causes lactic acidosis. Surg Endosc. 1998;12(3):198-201. doi:10.1007/s004649900633
53. Kashtan J, Green JF, Parsons EQ, Holcroft JW. Hemodynamic effects of increased abdominal pressure. J Surg Res. 1981;30(3):249-255. doi:10.1016/0022-4804(81)90156-6
54. Mutoh T, Lamm WJ, Embree LJ, Hildebrandt J, Albert RK. Abdominal distension alters regional pleural pressures and chest wall mechanics in pigs in vivo. J Appl Physiol (1985). 1991;70(6):2611-2618. doi:10.1152/jappl.1991.70.6.2611
55. El-Taji O, Howell-Etienne J, Taktak S, Hanchanale V. Lower vs standard pressure pneumoperitoneum in robotic-assisted radical prostatectomy: a systematic review and meta-analysis. J Robot Surg. 2023;17(2):303-312. doi:10.1007/s11701-022-01445-2
56. Abaza R, Ferroni MC. Randomized trial of ultralow vs standard pneumoperitoneum during robotic prostatectomy. J Urol. 2022;208(3):626-632. doi:10.1097/ju.0000000000002729
57. Huang X, Wang L, Zheng X, Wang X. Comparison of perioperative, functional, and oncologic outcomes between standard laparoscopic and robotic-assisted radical prostatectomy: a systemic review and meta-analysis. Surg Endosc. 2017;31(3):1045-1060. doi:10.1007/s00464-016-5125-1
58. Ficarra V, Novara G, Artibani W, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2009;55(5):1037-1063. doi:10.1016/j.eururo.2009.01.036
59. Porpiglia F, Morra I, Lucci Chiarissi M, et al. Randomised controlled trial comparing laparoscopic and robot-assisted radical prostatectomy. Eur Urol. 2013;63(4):606-614. doi:10.1016/j.eururo.2012.07.007
60. Chesnut GT, Benfante N, Barham D, et al. Routine postoperative hemoglobin assessment poorly predicts transfusion requirement among patients undergoing minimally invasive radical prostatectomy. Urol Pract. 2020;7(4):299-304. doi:10.1097/upj.0000000000000108
61. Joseph JP, Glasgow AE, Carlson RE, et al. Screening postoperative hemoglobin after robot-assisted radical prostatectomy-frequently used, but is it necessary? Urol Pract. 2020;7(6):554-558. doi:10.1097/UPJ.0000000000000121
62. Keating K, Rohloff M, Cicic A, Dehaan A, Maatman TJ. Are postoperative laboratory studies following robotic assisted radical prostatectomy necessary? Urol Pract. 2021;8(4):510-514. doi:10.1097/UPJ.0000000000000238
63. Chislett B, Omran G, Harvey M, Bolton D, Lawrentschuk N. Progressing towards same-day discharges after robotic-assisted radical prostatectomy; safe and cost effective to discharge without routine blood tests. Res Rep Urol. 2023;15:471-477. doi:10.2147/RRU.S429819
64. Martin AD, Nunez RN, Andrews JR, Martin GL, Andrews PE, Castle EP. Outpatient prostatectomy: too much too soon or just what the patient ordered. Urology. 2010;75(2):421-424. doi:10.1016/j.urology.2009.08.085
65. Abaza R, Martinez O, Ferroni MC, Bsatee A, Gerhard RS. Same day discharge after robotic radical prostatectomy. J Urol. 2019;202(5):959-963. doi:10.1097/JU.0000000000000353
66. Ploussard G, Dumonceau O, Thomas L, et al. Multi-institutional assessment of routine same day discharge surgery for robot-assisted radical prostatectomy. J Urol. 2020;204(5):956-961. doi:10.1097/ju.0000000000001129
67. Khalil MI, Bhandari NR, Payakachat N, Davis R, Raheem OA, Kamel MH. Perioperative mortality and morbidity of outpatient versus inpatient robot-assisted radical prostatectomy: a propensity matched analysis. Urol Oncol. 2020;38(1):3.e1-3.e6. doi:10.1016/j.urolonc.2019.07.008
68. Ploussard G, Grabia A, Barret E, et al. Same-day-discharge robot-assisted radical prostatectomy: an annual countrywide analysis. Eur Urol Open Sci. 2022;36:23-25. doi:10.1016/j.euros.2021.12.002
69. Cheng E, Gereta S, Zhang TR, et al. Same-day discharge vs inpatient robotic-assisted radical prostatectomy: complications, time-driven activity-based costing, and patient satisfaction. J Urol. 2023;210(6):856-864. doi:10.1097/JU.0000000000003678
70. Dobbs RW, Nguyen TT, Shahait M, et al. Outpatient robot-assisted radical prostatectomy: are patients ready for same-day discharge? J Endourol. 2020;34(4):450-455. doi:10.1089/end.2019.0796
71. Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78(5):606-617. doi:10.1093/bja/78.5.606
72. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152(3):292-298. doi:10.1001/jamasurg.2016.4952
73. Ye Z, Chen J, Shen T, et al. Enhanced recovery after surgery (ERAS) might be a standard care in radical prostatectomy: a systematic review and meta-analysis. Ann Palliat Med. 2020;9(3):746-758. doi:10.21037/apm.2020.04.03
74. Gomes T, Ledlie S, Tadrous M, Mamdani M, Paterson JM, Juurlink DN. Trends in opioid toxicity–related deaths in the us before and after the start of the COVID-19 pandemic, 2011-2021. JAMA Network Open. 2023;6(7):e2322303-e2322303. doi:10.1001/jamanetworkopen.2023.22303
75. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504. doi:10.1001/jamasurg.2017.0504
76. Patel HD, Faisal FA, Patel ND, et al. Effect of a prospective opioid reduction intervention on opioid prescribing and use after radical prostatectomy: results of the Opioid Reduction Intervention for Open, Laparoscopic, and Endoscopic Surgery (ORIOLES) Initiative. BJU Int. 2020;125(3):426-432. doi:10.1111/bju.14932
77. Su ZT, Becker REN, Huang MM, et al. Patient and in-hospital predictors of post-discharge opioid utilization: individualizing prescribing after radical prostatectomy based on the ORIOLES initiative. Urol Oncol. 2022;40(3):104 e9-104 e15. doi:10.1016/j.urolonc.2021.10.007
78. Shkolyar E, Shih I-F, Li Y, Wong JA, Liao JC. Robot-assisted radical prostatectomy associated with decreased persistent postoperative opioid use. J Endourol. 2020;34(4):475-481. doi:10.1089/end.2019.0788
79. Manning MW, Whittle J, Fuller M, et al. A multidisciplinary opioid-reduction pathway for robotic prostatectomy: outcomes at year one. Perioper Med. 2023;12(1):43. doi:10.1186/s13741-023-00331-1
80. Prebay ZJ, Medairos R, Landowski T, et al. Pain management following robotic-assisted radical prostatectomy: transitioning to an opioid free regimen. J Robot Surg. 2021;15(6):923-928. doi:10.1007/s11701-021-01191-x
81. Horodyski L, Ball B, Emile C, et al. Safe transition to opioid-free pathway after robotic-assisted laparoscopic prostatectomy. J Robot Surg. 2022;16(2):307-314. doi:10.1007/s11701-021-01237-0
82. Lee JE, Oh J, Lee JN, Ri H-S, Lee CS, Yeo J. Comparison of a non-opioid multimodal analgesia protocol with opioid-based patient-controlled analgesia for pain control following robot-assisted radical prostatectomy: a randomized, non-inferiority trial. J Pain Res. 2023;16:563-572. doi:10.2147/jpr.s397529
83. Hemal A. Opioid-free pain control regimen following robotic radical prostatectomy. ClinicalTrials.gov identifier: NCT05597878. Updated April 18, 2024. Accessed July 24, 2024. https://classic.clinicaltrials.gov/show/NCT05597878
84. Chenam A, Yuh B, Zhumkhawala A, et al. Prospective randomised non-inferiority trial of pelvic drain placement vs no pelvic drain placement after robot-assisted radical prostatectomy. BJU Int. 2018;121(3):357-364. doi:10.1111/bju.14010
85. Abaza R, Martinez O, Murphy C. Drains are not necessary in the majority of robot‐assisted urological procedures. BJU Int. 2022;129(2):162-163. doi:10.1111/bju.15634
86. Ma J, Chang Y, Xu W, et al. Pelvic drain placement after robot-assisted radical prostatectomy: meta-analysis. BJS Open. 2023;7(6):zrad143. doi:10.1093/bjsopen/zrad143
87. Huang MM, Patel HD, Su ZT, et al. A prospective comparative study of routine versus deferred pelvic drain placement after radical prostatectomy: impact on complications and opioid use. World J Urol. 2021;39(6):1845-1851. doi:10.1007/s00345-020-03439-x
88. Avulova S, Smith JA. Is comparison of robotic to open radical prostatectomy still relevant? Eur Urol. 2018;73(5):672-673. doi:10.1016/j.eururo.2018.01.011
ical prostatectomy performed with different robotic platforms: first comparative evidence between da Vinci and HUGO robot-assisted surgery robots. Eur Urol Focus. 2024;10(1):107-114. doi:10.1016/j.euf.2023.08.001
90. Alip S, Koukourikis P, Han WK, Rha KH, Na JC. Comparing Revo-i and da Vinci in Retzius-sparing robot-assisted radical prostatectomy: a preliminary propensity score analysis of outcomes. J Endourol. 2022;36(1):104-110. doi:10.1089/end.2021.0421
91. Chang KD, Abdel Raheem A, Choi YD, Chung BH, Rha KH. Retzius-sparing robot-assisted radical prostatectomy using the Revo-i robotic surgical system: surgical technique and results of the first human trial. BJU Int. 2018;122(3):441-448. doi:10.1111/bju.14245
92. Dibitetto F, Fede Spicchiale C, Castellucci R, et al. Extraperitoneal robot assisted laparoscopic prostatectomy with Versius system: single centre experience. Prostate Cancer Prostatic Dis. 2024;27(2):323-326. doi:10.1038/s41391-024-00810-6
93. Polom W, Matuszewski M. Initial experience of the Versius robotic system in robot-assisted radical prostatectomy: a study of 58 cases. Cent European J Urol. 2024;77(1):30-36. doi:10.5173/ceju.2023.241
Article Information
Published: September 13, 2024.
Author Contributions: Austin Drysch and Kathryn E. Fink were co–first authors. Hiten D. Patel and Ashley E. Ross were co–senior authors.
Conflict of Interest Disclosure: None.
Funding/Support: None.
Data Availability Statement: This article is a narrative review and does not contain any primary data. All data discussed in this review are derived from previously published studies, which are cited appropriately within the text.