Summary of Main Points
- In the ARASENS trial, darolutamide in combination with ADT and docetaxel substantially reduced the risk of death compared with ADT and docetaxel in men with metastatic HSPC.
- The most recent National Comprehensive Cancer Network guidelines support the use of triple-therapy regimens for men with high-volume metastatic HSPC.
- Multidisciplinary care alongside patient education and support are important to ensure that patients are willing to begin and remain on the chemotherapy component of the regimen and achieve optimal treatment outcomes, including prolonged survival, while maintaining an acceptable quality of life.
- Clinicians can consider darolutamide in combination with ADT and docetaxel as a viable treatment option for patients with metastatic HSPC.
Abbreviations
ADT androgen deprivation therapy
AE adverse event
ARANOTE Darolutamide in Addition to ADT Versus ADT in Metastatic Hormone-sensitive Prostate Cancer
ARASENS Darolutamide in Addition to Standard Androgen Deprivation Therapy and Docetaxel in Metastatic Hormone-Sensitive Prostate Cancer
ARSi androgen receptor signaling inhibitor
CHAARTED Chemo-Hormonal Therapy Versus Androgen Ablation Randomized Trial for Extensive Disease Prostate Cancer
CRPC castration-resistant prostate cancer
HR hazard ratio
HSPC hormone-sensitive prostate cancer
LATITUDE Study of Abiraterone Acetate Plus Low-Dose Prednisone Plus Androgen Deprivation Therapy (ADT) Versus ADT Alone in Newly Diagnosed Participants With High-Risk, Metastatic Hormone-Naïve Prostate Cancer
OS overall survival
PCa prostate cancer
PEACE1 Phase III Study for Patients With Metastatic Hormone-naïve Prostate Cancer
Prostate cancer (PCa) is the second-leading cause of cancer-related deaths among men in the United States, with an incidence of 128 per 100 000 men in 2020.1-3 It is estimated that 299 010 new cases will be diagnosed in 2024.3 Prostate cancer usually presents at an early stage and often follows an indolent course, but approximately one-third of men with localized disease experience disease progression and metastasis.4,5 Moreover, a small percentage of men present with de novo (newly diagnosed) metastatic PCa, and this proportion has increased over the past 20 years.2,6 Overall, metastatic PCa accounts for 8% of PCa cases.7 For men with metastatic PCa, the 5-year survival rate is 34%.7
The mainstay of treatment for metastatic hormone-sensitive PCa (HSPC) involves achieving castration levels of testosterone (<50 ng/mL)8,9 through surgery (bilateral orchidectomy) or androgen-deprivation therapy (ADT).10,11 There has been a recent focus on early treatment intensification through combination therapy for men with metastatic HSPC.10,12 Although ADT remains the cornerstone of treatment, studies have shown that the addition of a second-generation androgen receptor signaling inhibitor (ARSi) alone or with docetaxel chemotherapy (dual and triple therapy, respectively) confers a substantial survival benefit compared with ADT monotherapy.13 Dual therapy typically involves combining standard ADT with either docetaxel or an ARSi such as abiraterone, enzalutamide, or apalutamide, although the ADT-docetaxel combination is no longer considered the standard of care. Triple therapy involves the combination of ADT and docetaxel with the addition of an ARSi, such as abiraterone or darolutamide.
Darolutamide is a potent and structurally distinct ARSi approved for the treatment of metastatic HSPC by the US Food and Drug Administration in August 2022.14-16 In the Darolutamide in Addition to Standard Androgen Deprivation Therapy and Docetaxel in Metastatic Hormone-Sensitive Prostate Cancer (ARASENS) trial (ClinicalTrials.gov identifier NCT02799602), darolutamide added to ADT with docetaxel reduced the risk of death by 32.5% (hazard ratio [HR], 0.68 [95% CI, 0.57-0.80]; P < .001) vs ADT with docetaxel in men with metastatic HSPC.17
Metastatic HSPC is increasingly managed under a shared care model between urology and oncology specialists. This review describes the results of the ARASENS trial and the implications for care of men with metastatic HSPC to support oncologists’ and urologists’ clinical decision-making.
Evolving Treatment Landscape for Men With Metastatic HSPC
The treatment landscape of metastatic HSPC has evolved substantially over the past decade, with several therapeutic options beyond ADT. As the number of treatment options has increased, disease stratification in metastatic HSPC has gained increasing importance for assessing prognosis and guiding treatment decisions. Stratification is based on factors such as disease burden (number and location of metastases), timing of metastases, prostate-specific antigen levels, Gleason score, and performance status.18-22 More recently, there has been interest in the use of genomic predictors of outcomes, with studies showing that variations in genes such as TP53, RB1, and PTEN are associated with a poor prognosis.23,24 The current National Comprehensive Cancer Network guidelines encourage the use of ADT plus docetaxel in combination with either abiraterone or darolutamide for men with high-volume disease suitable for chemotherapy (Figure 1).25 The Chemohormonal Therapy Versus Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer (CHAARTED) criteria define high-volume disease as the presence of visceral metastases or 4 or more bone lesions, with at least 1 beyond the vertebral bodies and pelvis.18 Results from the CHAARTED study demonstrated that men with high-volume disease achieved a statistically significant improvement in overall survival (OS) with the addition of docetaxel to ADT. The criteria from the Study of Abiraterone Acetate Plus Low-Dose Prednisone Plus Androgen Deprivation Therapy (ADT) Versus ADT Alone in Newly Diagnosed Participants With High-Risk, Metastatic Hormone-Naïve Prostate Cancer (LATITUDE; ClinicalTrials.gov identifier NCT01715285) define high-risk disease as the presence of 2 or more of the following 3 factors: a Gleason score of 8 or higher, 3 or more bone lesions, and the presence of measurable visceral metastasis.19 The LATITUDE study enrolled men with de novo high-risk metastatic HSPC and demonstrated that these patients derived substantial benefit from dual therapy with abiraterone or prednisone plus ADT. Notably, studies have shown a partial concordance (87%) between the LATITUDE and CHAARTED criteria.26,27
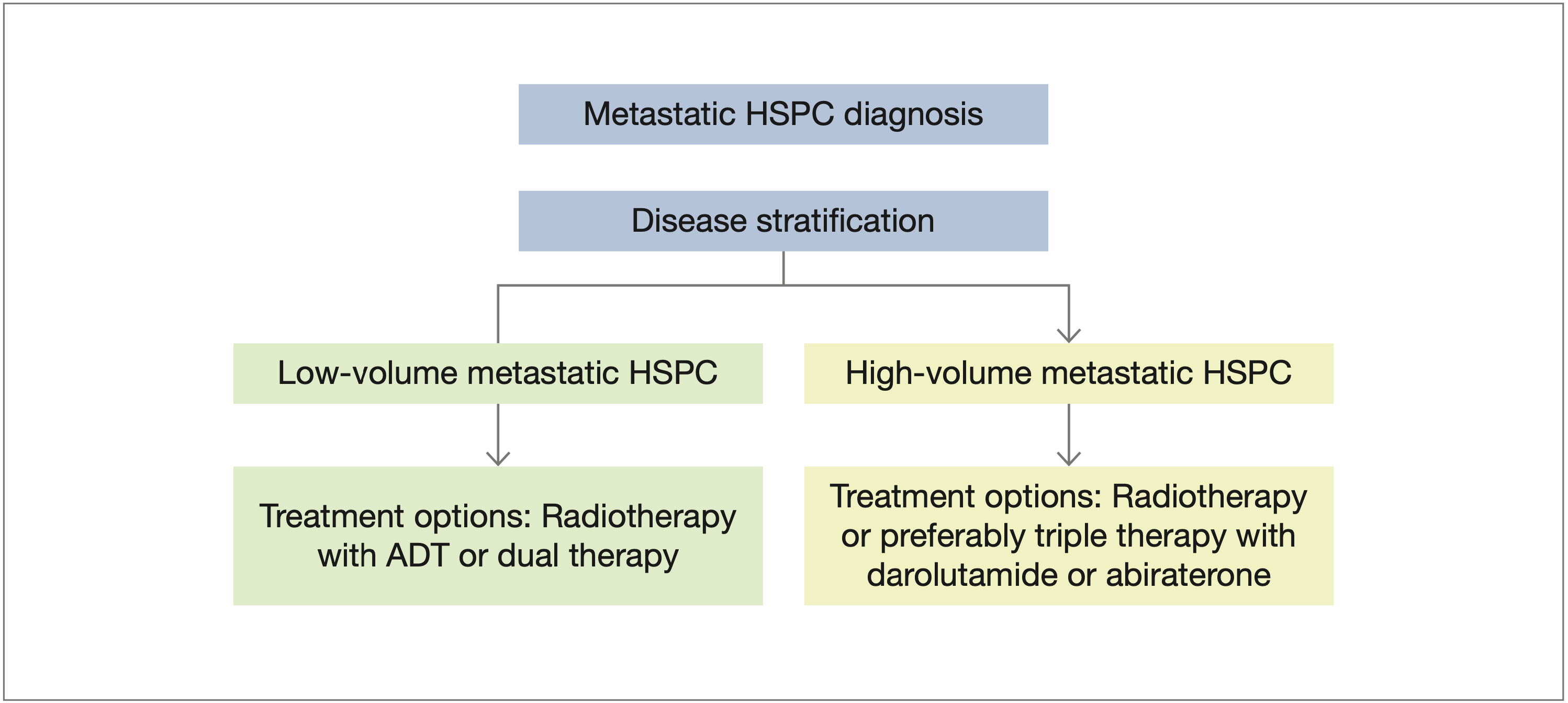
Figure 1. Treatment landscape in metastatic HSPC. The figure is based on the latest National Comprehensive Cancer Network treatment guidelines for patients with prostate cancer.25
Abbreviations: ADT, androgen-deprivation therapy; HSPC, hormone-sensitive prostate cancer.
Current Clinical Practice: A Collaboration Between Urology and Oncology
The treatment pathway for men with metastatic HSPC can vary widely between community and academic settings.28-31 Historically, academic medical centers have typically offered a wide range of specialized services, including comprehensive consulting and supportive care services, advanced treatments, and access to clinical trials and involvement in research.31,32 Academic settings often see more patients than other settings, including those with complex medical conditions. Moreover, academic settings have well-established coordination systems, with multidisciplinary teams and access to subspecialties.32 Academic centers are often located in urban areas, however, posing geographic barriers for some patients.33 Conversely, community settings focus on providing primary care and a broad range of general services to a local population, offering greater accessibility and convenience and potentially reducing patients’ travel and logistic challenges.31 These settings also enable the establishment of long-term patient-physician relationships, thereby fostering a personalized approach to care. Community settings may also have more limited resources and greater reliance on external referrals to access specialized care.31 In addition, the integration of advanced practice professionals and specialist nurses varies between practice types. More recently, large community-based urology groups have developed centers of expertise, providing a greater range of specialized cancer care, particularly through the use of advanced oncolytic agents. Collaborative efforts between community and academic settings, including referral systems and shared decision-making, can contribute to optimal patient care and treatment outcomes for men with metastatic HSPC.31,34
Co-Management of De Novo Metastatic HSPC by a Multidisciplinary Team
The management of de novo metastatic HSPC—where cancer has already metastasized at the time of initial diagnosis—requires a comprehensive approach involving a multidisciplinary team of health care professionals.35-37 This approach ensures that patients receive a holistic approach to their care. The multidisciplinary team may include urologists, medical oncologists, radiation oncologists, pathologists, diagnostic radiologists, advanced practice professionals, nurse navigators, and other specialists working collaboratively to determine the most suitable treatment plan for each patient.36,38 By taking advantage of the expertise of various specialists, the team can better diagnose and stage the disease and can make informed decisions about optimal treatment selection as well as treatment sequencing and timing, leading to improved patient outcomes and quality of life.37 An important part of the collaborative approach is for all multidisciplinary team members to have the necessary knowledge and confidence to help patients to choose the right treatment option and effectively manage their subsequent care.
Darolutamide Clinical Development
Darolutamide, developed by Orion Pharma and Bayer Healthcare Pharmaceuticals, is the latest treatment for metastatic HSPC. It is an androgen receptor antagonist that competitively inhibits androgens from binding to their receptors, thus inhibiting receptor nuclear translocation and transcriptional activity. This inhibition leads to a decrease in PCa cell proliferation and tumor volume.15
Darolutamide is structurally distinct from apalutamide and enzalutamide, with greater flexibility and higher polarity. In competitive androgen receptor binding assays, darolutamide showed tighter binding to the androgen receptor than apalutamide or enzalutamide.15 The greater flexibility, higher polarity, and greater hydrogen bond-forming potential of darolutamide are associated with low blood-brain barrier penetration.39,40 Low penetration of the blood-brain barrier may lead to a low potential for central nervous system–related adverse effects.41,42 In preclinical trials, darolutamide showed statistically significantly lower penetration of the blood-brain barrier (2%-4%) than apalutamide (29.3%) and enzalutamide (18.8%).15,43,44 The preclinical and early-phase Safety and Pharmacokinetics Study of ODM-201 in Castrate Resistant Prostate Cancer (ARADES) trials (ClinicalTrials.gov identifiers NCT01317641 [original trial] and NCT01429064 [12-week follow-up]) demonstrated antitumor activity and a favorable safety profile for darolutamide in men with metastatic castration-resistant PCa (CRPC).45,46 Similarly, the phase 1 Bioavailability Study of ODM-201 in Subjects With metastatic Chemotherapy-naïve Castration-resistant Prostate Cancer (ARAFOR) trial (ClinicalTrials.gov identifier NCT01784757), which investigated the long-term safety and tolerability of darolutamide in men with metastatic CRPC, reported a favorable safety and tolerability profile for darolutamide.47 The phase 3 Efficacy and Safety Study of Darolutamide (ODM-201) in Men With High-risk Non-metastatic Castration-resistant Prostate Cancer (ARAMIS) trial (ClinicalTrials.gov identifier NCT02200614) investigated the efficacy of darolutamide and showed improvement in metastasis-free survival compared with placebo for men with nonmetastatic CRPC and a rapid prostate-specific antigen doubling time.48 The ARASENS trial was an international, randomized, double-blind, placebo-controlled, phase 3 trial in men with metastatic HSPC. The results showed that the combination of darolutamide, ADT, and docetaxel increased OS compared with ADT and docetaxel alone in this patient population.17
Darolutamide in the Management of Metastatic HSPC
The combination of darolutamide, ADT, and docetaxel offers complementary mechanisms of action. As an ARSi with high binding affinity and selectivity for the androgen receptor, darolutamide prevents androgen receptor signaling,15 ADT reduces the production of androgens,49 and docetaxel inhibits microtubule function and disrupts cell division.50 The synergistic effects of these agents can lead to a more comprehensive and effective suppression of PCa cell growth.
Results from the ARASENS trial demonstrated that the addition of darolutamide to ADT and docetaxel statistically significantly increased OS in men with metastatic HSPC.17 Moreover, the similar frequencies of adverse events (AEs) in the darolutamide and placebo groups suggest that the treatment has an acceptable risk-benefit profile. A post hoc analysis of ARASENS reported the impact of disease volume and risk on efficacy and safety outcomes in men with metastatic HSPC.51 High-volume disease was defined according to the CHAARTED definition, and high-risk disease was defined according to the LATITUDE definition. Darolutamide increased OS compared with placebo in men with high-volume disease (HR, 0.69 [95% CI, 0.57-0.82]), high-risk disease (HR, 0.71 [95% CI, 0.58-0.86]), and low-risk disease (HR, 0.62 [95% CI, 0.42-0.90]), and results in the small subgroup of men with low-volume disease (HR, 0.68 [95% CI, 0.41-1.13]) also suggested a survival benefit for darolutamide. Across all subgroups, benefits of darolutamide were observed in terms of time to CRPC and subsequent systemic antineoplastic therapy.51 It is important to note that these trials administered 6 cycles of docetaxel, but darolutamide and ADT were administered over a 4-year period. Most of the AEs observed in the ARASENS study were the result of known toxicities associated with docetaxel treatment and were generally transient and manageable.17,51
The application of triple therapy (darolutamide plus ADT and docetaxel) has benefits for clinical practice, including expansion of the treatment options available for men with metastatic HSPC. This expansion allows more tailored approaches to individual patients. Overall, the trial results for darolutamide have notable implications for real-world clinical practice and support the use of darolutamide in combination with ADT and docetaxel as a potential new standard of care for men with metastatic HSPC.25,52
Clinical Considerations for Triple Therapy
Health care professionals often use shared decision-making to tailor treatment plans and provide therapies for metastatic HSPC based on the individual characteristics and disease burden of patients.
Traditionally, ADT monotherapy has been the standard treatment for metastatic HSPC, but several recent clinical trials have shown that combination therapy confers substantial survival benefit compared with ADT alone (Table 1).
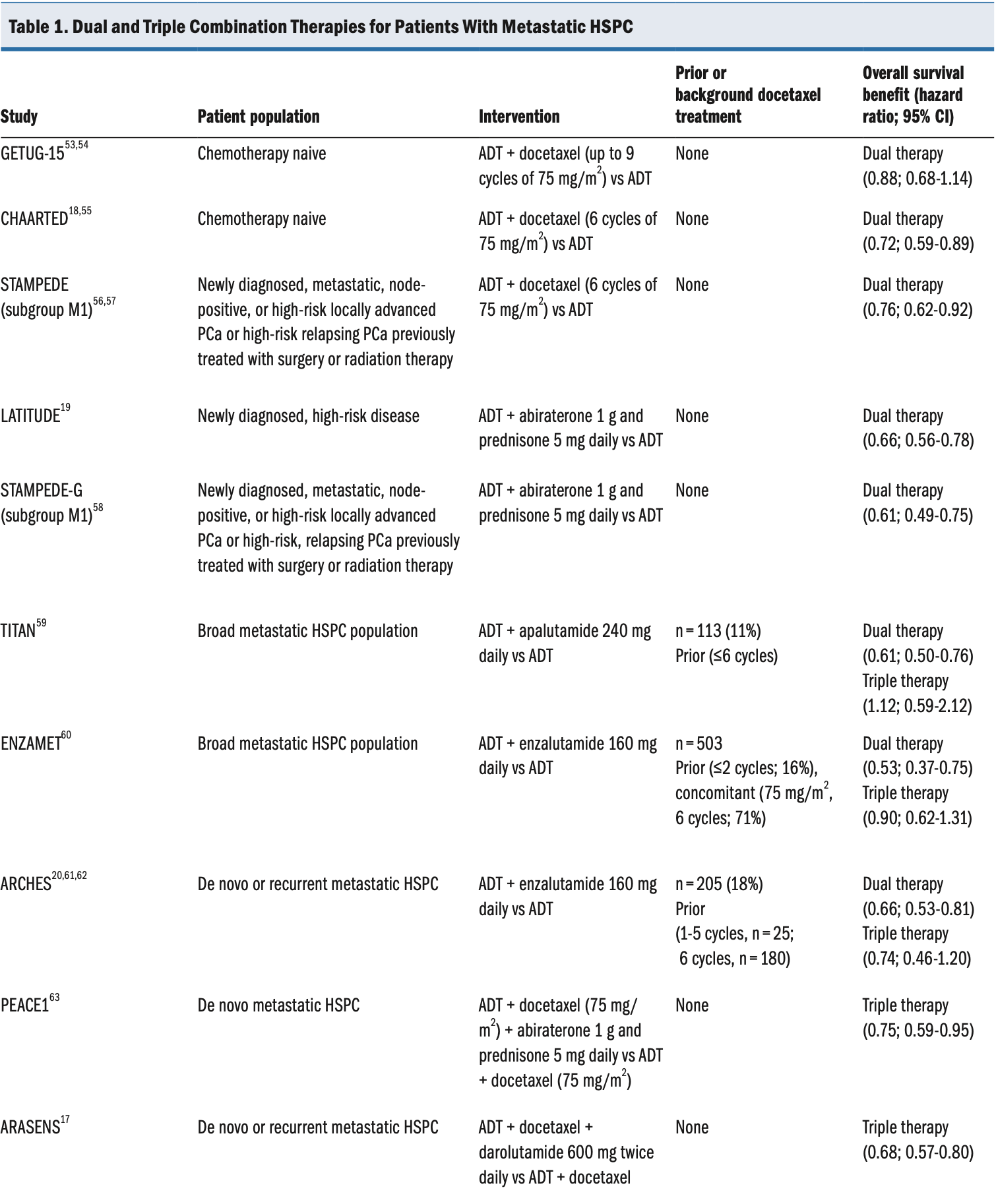
Abbreviations: ADT, androgen-deprivation therapy; HSPC, hormone-sensitive prostate cancer; PCa, prostate cancer.
More recently, following the results of the Phase III Study for Patients With Metastatic Hormone-naïve Prostate Cancer (PEACE1; ClinicalTrials.gov identifier NCT01957436) and ARASENS trials, the National Comprehensive Cancer Network guidelines were updated to support the use of triple-therapy regimens for men with high-volume, metastatic HSPC.25
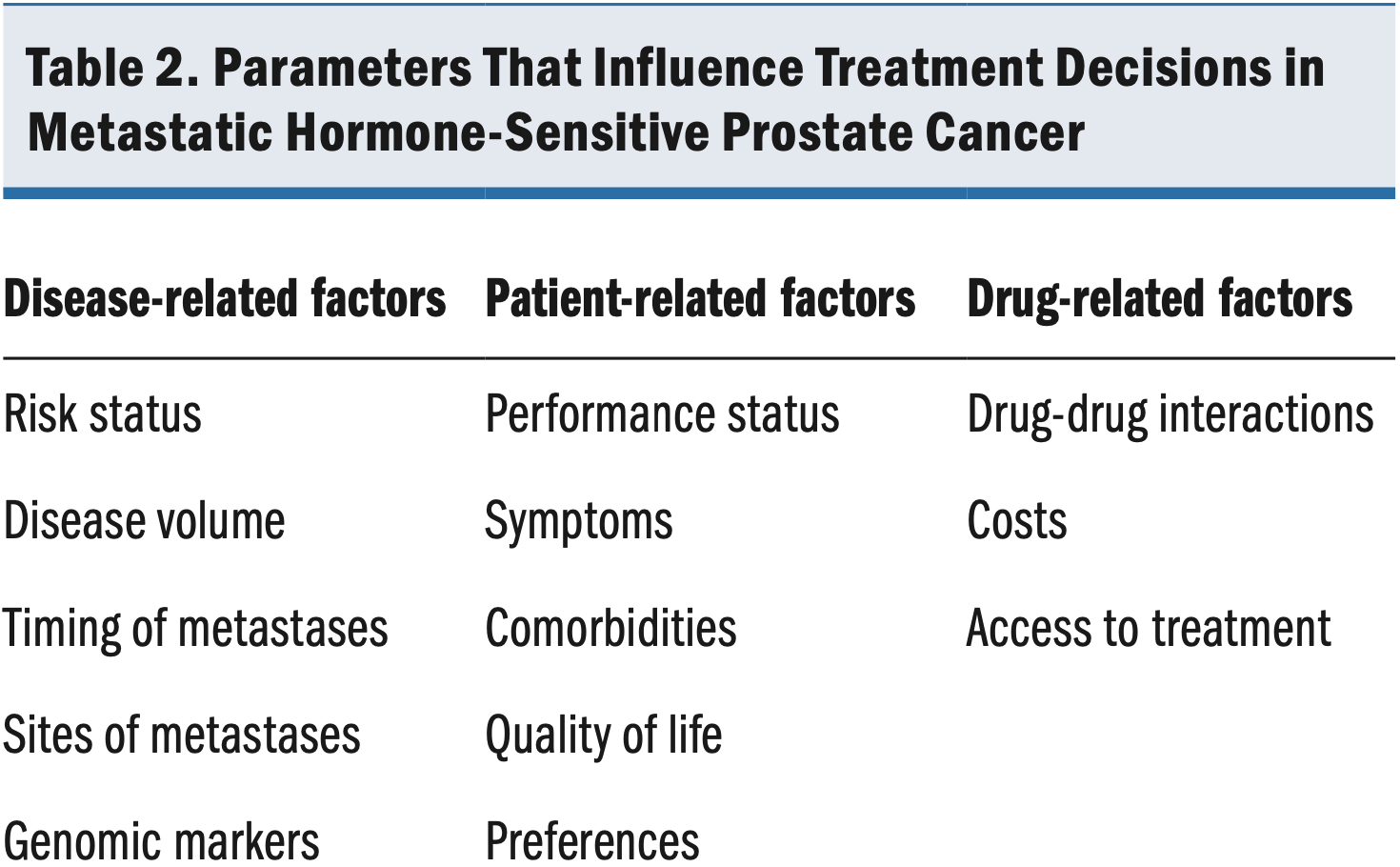
A wide range of disease-, patient-, and drug-related factors must be taken into account when one is considering therapy for metastatic HSPC (Table 2). Key disease-related factors include risk status, volume, timing of metastases, and genomics. For example, men with high-volume, high-risk metastatic HSPC have been reported to benefit more from triple therapy than do men with low-volume, low-risk disease.51,63 Core patient-related factors include performance status, symptoms, and comorbidities as well as any treatment preferences the patient expresses. Factors such as the presence of an active infection; preexisting neuropathy, which may worsen with chemotherapy64; and heart failure, where chemotherapy-induced fluid retention65 can exacerbate the condition, warrant caution when starting the chemotherapy treatment.66,67 In addition, patients with an existing fistula or who are at high risk of developing it, patients with a high risk of infection, and patients with liver or lung disease may not be suitable for chemotherapy.67-69 Individual assessment, considering overall health and the potential benefits and risks, is essential in determining whether chemotherapy is appropriate for an individual patient. Therefore, close collaboration between patients and clinicians is essential to making informed treatment decisions.
Managing Concerns About Chemotherapy for Metastatic HSPC
Docetaxel binds to microtubules, causing cell-cycle arrest and apoptosis.50 This analog of paclitaxel was first approved by the Food and Drug Administration in May 1996.67 Since then, it has been used widely in various chemotherapy regimens, contributing to improved outcomes for patients with a variety of malignancies. The CHAARTED trial investigated the combination of ADT with 6 cycles of docetaxel vs ADT alone in men with metastatic HSPC. The combination increased OS by a median of 10.4 months (HR, 0.72 [95% CI, 0.59-0.89]; P = .0018).18 Several other trials have also validated the efficacy of docetaxel directly56 or indirectly63,70 in men with metastatic HSPC. Because the evidence supporting the use of docetaxel in metastatic HSPC is derived primarily from clinical trials, however, clinicians may have reservations about translating the trial results to real-world practice.
Historically, the most worrisome aspect of docetaxel and chemotherapeutic drugs for patients has generally been the side effects, including nausea, vomiting, diarrhea, fatigue, edema, cytopenia, infections, and hair loss. In addition, docetaxel can cause neutropenia and increase the risk of infection and sepsis, which is of particular concern in light of emergent diseases such as COVID-19. When discussing treatment options with patients, it is important to note that not all patients experience the same side effects, and advancements in supportive care therapies have substantially mitigated these effects. For example, antinausea medications are effective, allowing patients to manage and even prevent nausea and vomiting,71 and cooling caps have been introduced to minimize hair loss during the treatment.72 Moreover, lifestyle adjustments, such as regular exercise and balanced diet, have proven valuable in managing chemotherapy-induced fatigue.73
In ARASENS, only 12% of patients were unable to complete the 6 cycles of docetaxel17; the majority of the patients completed the full treatment course, underscoring the tolerability and effectiveness of docetaxel treatment for men with metastatic HSPC.
Managing Patient Expectations Through Education and Support
Patient education is crucial for enabling patients to make informed decisions about their treatment. Many patients research their condition on the internet and have fears about what they have read or experienced through family or friends with cancer. One key role of physicians, including urologists—often the referring professional for chemotherapy—is to help patients interpret what they have learned and provide respectful, honest, reliable, and consistent information. Advanced practice professionals and nurses also play a critical role here, helping patients develop reasonable expectations, explaining that most of the side effects are temporary and manageable, which in turn helps the multidisciplinary team deliver the best care possible.
Health care professionals should engage in shared decision-making with patients and their caregivers, discussing the potential benefits, risks, and outcomes of triple therapy with darolutamide or abiraterone. Patients also need to be aware that managing cancer with chemotherapy can potentially improve their quality of life by reducing cancer-related symptoms and preventing or at least delaying disease progression. Patient support groups and counseling services can also offer patients an opportunity to connect with other patients going through similar experiences and receive emotional support.
Expert Experience With Triple Therapy in Practice
In practice, a key feature of darolutamide is its ease of administration, with a recommended twice-daily dose of 600 mg, taken with food, and no need for treatment monitoring.14 In addition, the toxicity profile of darolutamide is manageable; the most common AEs are increased aspartate aminotransferase and bilirubin levels, decreased neutrophil count, fatigue, pain in the extremities, and rash. For patients experiencing at least grade 3 AEs, moderate or severe liver disease, or severe kidney impairment, dosage reduction to 300 mg twice daily is advised. Dosage reduction below that level is not recommended.14
Drug-drug interactions with darolutamide are generally limited, although coadministration of darolutamide with drugs that induce both P-glycoprotein and cytochrome CYP3A4 should be avoided.14 Coadministration with drugs that inhibit both P-glycoprotein and cytochrome CYP3A4 may lead to increased darolutamide exposure; therefore, patients also receiving such agents should be monitored more closely for emergent AEs.
Overall, the combination of ease of administration, lack of a need for ongoing monitoring, low toxicity, and few interactions with other drugs enhances and simplifies the treatment experience for patients and their treatment team.
Discussion
The results from the ARASENS trial showed that the combination of darolutamide with ADT and docetaxel was associated with a statistically significant OS increase in men with high-volume disease.17 These results build on those of the PEACE1 trial, which demonstrated the advantage of triple therapy over dual therapy with ADT plus docetaxel.63 Importantly, the consistency of the survival benefits in ARASENS and PEACE1 reinforces the notion that intensified treatment with triple therapy can offer superior outcomes for men with metastatic HSPC. In addition, the similar frequency of AEs between the darolutamide and placebo groups in ARASENS suggests that the treatment has an acceptable safety profile. Real-world data regarding triple therapy with darolutamide are limited, however, emphasizing the need for further research and validation in clinical practice to fully realize the benefits of this treatment regimen. Further data on the safety and efficacy of darolutamide in men with PCa are expected from 3 ongoing clinical trials. In the phase 3 Darolutamide in Addition to ADT Versus ADT in Metastatic Hormone-sensitive Prostate Cancer (ARANOTE) trial (ClinicalTrials.gov identifier NCT04736199), darolutamide plus ADT vs ADT alone is being investigated in men with metastatic HSPC globally (excluding the United States). The phase 2 Study to Learn How Well Darolutamide Administered Together With Androgen Deprivation Therapy (ADT) Works in Men With Metastatic Hormone-sensitive Prostate Cancer. Results Will be Compared With ADT Alone From a Previously Conducted Study (ARASEC) trial (ClinicalTrials.gov identifier NCT05059236) will complement ARANOTE by evaluating darolutamide plus ADT vs ADT alone in US patients. Finally, the phase 3 Study to Compare Darolutamide Given With Androgen Deprivation Therapy (ADT) With ADT in Men With Hormone Sensitive Prostate Cancer and Raise of Prostate Specific Antigen (PSA) Levels After Local Therapies (ARASTEP) trial (ClinicalTrials.gov identifier NCT05794906) aims to compare darolutamide plus ADT vs ADT alone in men with nonmetastatic PCa.
Conclusion
The results of the ARASENS trial offer a new treatment modality for patients with metastatic HSPC, showing a statistically significant reduction in risk of death with the addition of darolutamide to ADT plus docetaxel. Men with metastatic HSPC may achieve prolonged survival while maintaining an acceptable quality of life. Health care professionals can consider the use of darolutamide in combination with ADT and docetaxel as a viable treatment option for patients with metastatic HSPC.
References
1. Siegel R, Miller K, Fuchs H, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi:10.3322/caac.21708
2. Wang L, Lu B, He M, et al. Prostate cancer incidence and mortality: global status and temporal trends in 89 countries from 2000 to 2019. Front Public Health. 2022;10:811044. doi:10.3389/fpubh.2022.811044
3. American Cancer Society. Key statistics for prostate cancer. Accessed February 19, 2024. https://www.cancer.org/cancer/types/prostate-cancer/about/key-statistics.html
4. Ward JF, Blute ML, Slezak J, Bergstralh EJ, Zincke H. The long-term clinical impact of biochemical recurrence of prostate cancer 5 or more years after radical prostatectomy. J Urol. 2003;170(5):1872-1876. doi:10.1097/01.ju.0000091876.13656.2e
5. Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591-1597. doi:10.1001/jama.281.17.1591
6. Zhang AC, Rasul R, Golden A, Feuerstein MA. Incidence and mortality trends of metastatic prostate cancer: surveillance, epidemiology, and end results database analysis. Can Urol Assoc J. 2021;15(12):E637-E643. doi:10.5489/cuaj.7173
7. Howlander N, Noone A, Krapcho M, et al. SEER cancer statistics review, 1975-2018. National Cancer Institute. 2021. Accessed May 7, 2024. https://seer.cancer.gov/csr/1975_2018/
8. Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34(12):1402-1418. doi:10.1200/JCO.2015.64.2702
9. Kanesvaran R, Castro E, Wong A, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with prostate cancer. ESMO Open. 2022;7(4):100518. doi:10.1016/j.esmoop.2022.100518
10. Virgo KS, Rumble RB, de Wit R, et al. Initial management of noncastrate advanced, recurrent, or metastatic prostate cancer: ASCO guideline update. J Clin Oncol. 2021;39(11):1274-1305. doi:10.1200/JCO.20.03256
11. Desmond A, Arnold A, Hastie K. Subcapsular orchiectomy under local anaesthesia technique, results and implications. B J Urol. 1988;61(2):143-145. doi:10.1111/j.1464-410x.1988.tb05063.x
12. McKay RR. Treatment intensification for low-risk metastatic hormone-sensitive prostate cancer: the time is now. Eur Urol Open Sci. 2022;45:41-43. doi:10.1016/j.euros.2022.09.009
13. Sathianathen NJ, Koschel S, Thangasamy IA, et al. Indirect comparisons of efficacy between combination approaches in metastatic hormone-sensitive prostate cancer: a systematic review and network meta-analysis. Eur Urol. 2020;77(3):365-372. doi:10.1016/j.eururo.2019.09.004
14. NUBEQA™ (darolutamide). US prescribing information. Bayer HealthCare Pharmaceuticals Inc; 2022. Accessed May 7, 2024. https://labeling.bayerhealthcare.com/html/products/pi/Nubeqa_PI.pdf
15. Moilanen AM, Riikonen R, Oksala R, et al. Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci Rep. 2015;5:12007. doi:10.1038/srep12007
16. Orion Corporation. US FDA approves additional indication of darolutamide in combination with docetaxel for the treatment of metastatic hormone-sensitive prostate cancer (mHSPC). GlobeNewswire. 2022. Accessed May 7, 2024. https://mfn.se/one/a/orion/u-s-fda-approves-additional-indication-of-darolutamide-in-combination-with-docetaxel-for-the-treatment-of-metastatic-hormone-sensitive-prostate-cancer-mhspc-6fcef424
17. Smith MR, Hussain M, Saad F, et al. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. 2022;386(12):1132-1142. doi:10.1056/NEJMoa2119115
18. Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36(11):1080-1087. doi:10.1200/JCO.2017.75.3657
19. Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686-700. doi:10.1016/S1470-2045(19)30082-8
20. Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974-2986. doi:10.1200/JCO.19.00799
21. Rodrigues G, Warde P, Pickles T, et al. Pre-treatment risk stratification of prostate cancer patients: a critical review. Can Urol Assoc J. 2012;6(2):121-127. doi:10.5489/cuaj.11085
22. Frees S, Akamatsu S, Bidnur S, et al. The impact of time to metastasis on overall survival in patients with prostate cancer. World J Urol. 2018;36(7):1039-1046. doi:10.1007/s00345-018-2236-4
23. Bostrom PJ, Bjartell AS, Catto JW, et al. Genomic predictors of outcome in prostate cancer. Eur Urol. 2015;68(6):1033-1044. doi:10.1016/j.eururo.2015.04.008
24. Bang S, Won D, Shin S, et al. Circulating tumor DNA analysis on metastatic prostate cancer with disease progression. Cancers (Basel). 2023;15(15):3998. doi:10.3390/cancers1515399
25. Schaeffer EM, Srinivas S, Adra N, et al. NCCN Guidelines® insights: prostate cancer, version 1.2023: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2022;20(12):1288-1298. doi:10.6004/jnccn.2022.0063
26. Iacovelli R, Ciccarese C, Mosillo C, et al. Comparison between prognostic classifications in de novo metastatic hormone sensitive prostate cancer. Target Oncol. 2018;13(5):649-655. doi:10.1007/s11523-018-0588-8
27. Kawahara T, Yoneyama S, Ohno Y, et al. Prognostic value of the LATITUDE and CHAARTED risk criteria for predicting the survival of men with bone metastatic hormone-naive prostate cancer treated with combined androgen blockade therapy: real-world data from a Japanese multi-institutional study. Biomed Res Int. 2020;2020:7804932. doi:10.1155/2020/7804932
28. Cooperberg MR, Broering JM, Litwin MS, et al. The contemporary management of prostate cancer in the United States: lessons from the cancer of the prostate strategic urologic research endeavor (CapSURE), a national disease registry. J Urol. 2004;171(4):1393-1401. doi:10.1097/01.ju.0000107247.81471.06
29. Sartor O, Appukkuttan S, Weiss J, Tsao CK. Clinical outcomes, management, and treatment patterns in patients with metastatic castration‐resistant prostate cancer treated with radium‐223 in community compared to academic settings. Prostate. 2021;81(10):657-666. doi:10.1002/pros.24143
30. Mahal BA, Chen YW, Muralidhar V, et al. National sociodemographic disparities in the treatment of high‐risk prostate cancer: do academic cancer centers perform better than community cancer centers? Cancer. 2016;122(21):3371-3377. doi:10.1002/cncr.30205
31. Desch CE, Blayney DW. Making the choice between academic oncology and community practice: the big picture and details about each career. J Oncol Pract. 2006;2(3):132-136. doi:10.1200/JOP.2006.2.3.132
32. Wartman S. The Transformation of Academic Health Centers: Meeting the Challenges of Healthcare’s Changing Landscape. Academic Press; 2015. Accessed May 7, 2024. https://books.google.co.uk/books?hl=en&lr=&id=oOKcBAAAQBAJ&oi=fnd&pg=PP1&dq=Wartman+S.+The+transformation+of+academic+health+centers:+meeting+the+challenges+of+healthcare%E2%80%99s+changing+landscape.+Cambridge,+MA,+Academic+Press,+2015.&ots=opB1XgXzCf&s
33. Bilimoria KY, Ko CY, Tomlinson JS, et al. Wait times for cancer surgery in the United States: trends and predictors of delays. Ann Surg. 2011;253(4):779-785. doi:10.1097/SLA.0b013e318211cc0f
34. Melas M, Subbiah S, Saadat S, Rajurkar S, McDonnell KJ. The community oncology and academic medical center alliance in the age of precision medicine: cancer genetics and genomics considerations. J Clin Med. 2020;9(7):2125. doi:10.3390/jcm9072125
35. Weiner AB, Nettey OS, Morgans AK. Management of metastatic hormone-sensitive prostate cancer (mHSPC): an evolving treatment paradigm. Curr Treat Options Oncol. 2019;20(9):69. doi:10.1007/s11864-019-0668-8
36. Montagut C, Albanell J, Bellmunt J. Prostate cancer. Multidisciplinary approach: a key to success. Crit Rev Oncol Hematol. 2008;68(1):S32-S36.
37. Thomas CR Jr. Prostate Cancer: A Multidisciplinary Approach to Diagnosis and Management. Demos Medical; 2014.
38. Sternberg CN, Krainer M, Oh WK, et al. The medical management of prostate cancer: a multidisciplinary team approach. BJU Int. 2007;99(1):22-27. doi:10.1111/j.1464-410X.2006.06477.x
39. Pajouhesh H, Lenz GR. Medicinal chemical properties of successful central nervous system drugs. NeuroRx. 2005;2(4):541-553. doi:10.1602/neurorx.2.4.541
40. Darolutamide. PubChem compound summary for CID 67171867. National Center for Biotechnology Information; 2023. Accessed May 7, 2024. https://pubchem.ncbi.nlm.nih.gov/compound/Darolutamide
41. Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235-1246. doi:10.1056/NEJMoa1815671
42. Fizazi K, Shore N, Tammela TL, et al. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med. 2020;383(11):1040-1049. doi:10.1056/NEJMoa2001342
43. Clegg NJ, Wongvipat J, Joseph JD, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72(6):1494-1503. doi:10.1158/0008-5472.CAN-11-3948
44. Zurth C, Sandman S, Trummel D, et al. Higher blood–brain barrier penetration of [14C] apalutamide and [14C] enzalutamide compared to [14C] darolutamide in rats using whole-body autoradiography. J Clin Oncol. 2019;37. doi:10.1200/JCO.2019.37.7_suppl.156
45. Fizazi K, Massard C, Bono P, et al. Activity and safety of ODM-201 in patients with progressive metastatic castration-resistant prostate cancer (ARADES): an open-label phase 1 dose-escalation and randomised phase 2 dose expansion trial. Lancet Oncol. 2014;15(9):975-985. doi:10.1016/S1470-2045(14)70240-2
46. Fizazi K, Massard C, Bono P, et al. Safety and antitumour activity of ODM-201 (BAY-1841788) in castration-resistant, CYP17 inhibitor-naïve prostate cancer: results from extended follow-up of the ARADES trial. Eur Urol Focus. 2017;3(6):606-614. doi:10.1016/j.euf.2017.01.010
47. Massard C, Penttinen HM, Vjaters E, et al. Pharmacokinetics, antitumor activity, and safety of ODM-201 in patients with chemotherapy-naive metastatic castration-resistant prostate cancer: an open-label phase 1 study. Eur Urol. 2016;69(5):834-840. doi:10.1016/j.eururo.2015.09.046
48. Fizazi K, Shore N, Smith M, et al. 633P Tolerability and treatment response to darolutamide (DARO) in patients with non-metastatic castration-resistant prostate cancer (nmCRPC) in the phase III ARAMIS trial. Ann Oncol. 2020;31:S522. doi:10.1016/j.annonc.2020.08.892
49. Mohler JL. Concept and viability of androgen annihilation for advanced prostate cancer. Cancer. 2014;120(17):2628-2637. doi:10.1002/cncr.28675
50. Herbst RS, Khuri FR. Mode of action of docetaxel—a basis for combination with novel anticancer agents. Cancer Treat Rev. 2003;29(5):407-415. doi:10.1016/s0305-7372(03)00097-5
51. Hussain M, Tombal B, Saad F, et al. Darolutamide plus androgen-deprivation therapy and docetaxel in metastatic hormone-sensitive prostate cancer by disease volume and risk subgroups in the phase III ARASENS trial. J Clin Oncol. 2023;41(20)3595-3607. doi:10.1200/JCO.23.00041
52. Hack J, Crabb SJ. Is triple therapy the new standard for metastatic hormone-sensitive prostate cancer? Oncol Haematol. 2022;18:120-124. doi:10.17925/OHR.2022.18.2.120
53. Gravis G, Boher JM, Joly F, et al. Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 trial. Eur Urol. 2016;70(2):256-262. doi:10.1016/j.eururo.2015.11.005
54. Gravis G, Fizazi K, Joly F, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(2):149-158. doi:10.1016/S1470-2045(12)70560-0
55. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746. doi:10.1056/NEJMoa1503747
56. James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177. doi:10.1016/S0140-6736(15)01037-5
57. James ND, Sydes MR, Clarke NW, et al. Systemic therapy for advancing or metastatic prostate cancer (STAMPEDE): a multi‐arm, multistage randomized controlled trial. BJU Int. 2009;103(4):464-469. doi:10.1111/j.1464-410X.2008.08034.x
58. James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377(4):338-351. doi:10.1056/NEJMoa1702900
59. Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. 2021;39(20):2294-2303. doi:10.1200/JCO.20.03488
60. Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121-131. doi:10.1056/NEJMoa1903835
61. Armstrong AJ, Iguchi T, Azad AA, et al. The efficacy of enzalutamide plus androgen deprivation therapy in oligometastatic hormone-sensitive prostate cancer: a post hoc analysis of ARCHES. Eur Urol. 2023;84(2):229-241. doi:10.1016/j.eururo.2023.04.002
62. Tsaur I, Mirvald C, Surcel C. Triple therapy in metastatic hormone-sensitive prostate cancer. Curr Opin Urol. 2023;33(6):452-457. doi:10.1097/MOU.0000000000001125
63. Fizazi K, Foulon S, Carles J, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet. 2022;399(10336):1695-1707. doi:10.1016/S0140-6736(22)00367-1
64. Windebank AJ, Grisold W. Chemotherapy‐induced neuropathy. J Peripher Nerv Syst. 2008;13(1):27-46. doi:10.1111/j.1529-8027.2008.00156.x
65. Mase T, Honda S, Yamano M, Kawasaki T. A case of docetaxel-induced left ventricular outflow tract obstruction. Cureus. 2023;15(8):e43598. doi:10.7759/cureus.43598
66. Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments: what the cardiologist needs to know. Nat Rev Cardiol. 2010;7(10):564-575. doi:10.1038/nrcardio.2010.121
67. Taxotere. US prescribing information. Sanofi-Aventis; 2021. Accessed May 7, 2024. https://products.sanofi.us/taxotere/taxotere.html
68. Anido-Herranz U, Fernandez-Nunez N, Afonso-Afonso J, et al. Chemotherapy management for unfit patients with metastatic castration-resistant prostate cancer. Clin Transl Oncol. 2019;21(3):249-258. doi:10.1007/s12094-018-1928-y
69. Bodey GP. Infections in cancer patients. Cancer Treat Rev. 1975;2(2):89-128. doi:10.1016/s0305-7372(75)80005-3
70. Smith MR, Hussain MH, Saad F, et al. Overall survival with darolutamide versus placebo in combination with androgen-deprivation therapy and docetaxel for metastatic hormone-sensitive prostate cancer in the phase 3 ARASENS trial. J Clin Oncol. 2022;40(suppl 6):13. doi:10.1200/JCO.2022.40.6_suppl.013
71. Rao KV, Faso A. Chemotherapy-induced nausea and vomiting: optimizing prevention and management. Am Health Drug Benefits. 2012;5(4):232-240.
72. Massey CS. A multicentre study to determine the efficacy and patient acceptability of the Paxman Scalp Cooler to prevent hair loss in patients receiving chemotherapy. Eur J Oncol Nurs. 2004;8(2):121-130. doi:10.1016/j.ejon.2003.10.006
73. Bourke L, Gilbert S, Hooper R, et al. Lifestyle changes for improving disease-specific quality of life in sedentary men on long-term androgen-deprivation therapy for advanced prostate cancer: a randomised controlled trial. Eur Urol. 2014;65(5):865-872. doi:10.1016/j.eururo.2013.09.040
Article Information
Published: x.
Author Contributions: All authors participated equally in concept and design, manuscript review, and final approval of the manuscript, and are accountable for all aspects of the work.
Conflict of Interest Disclosures: Paul Dato has received consultant or advisory role fees from Astellas, Bayer, Dendreon, Janssen, and Pfizer as well as speaker fees from Astellas, Bayer, Dendreon, Exact Imaging, Janssen, Myovant, and Pfizer. Rana R. McKay has received consultant or advisory role fees from AstraZeneca, Aveo, Bayer, Bristol Myers Squibb, Calithera, Caris, Dendreon, Eisai, Eli Lilly, Exelixis, Janssen, Merck, Myovant, Novartis, Pfizer, Sanofi, SeaGen, Sorrento Therapeutics, Telix, and Tempus as well as research support or funding from Artera, AstraZeneca, Bayer, Bristol Myers Squibb, Exelixis, Oncternal, and Tempus.
Funding/Support: The ARASENS study was funded by Bayer HealthCare Pharmaceuticals, US, supported by Bayer HealthCare Pharmaceuticals, US. The funders had no role in the preparation, review, or approval of the article or the decision to submit the article for publication. Any opinions, findings, or conclusions expressed in this material are those of the authors and do not necessarily reflect those of Bayer HealthCare Pharmaceuticals, US.
Data Availability Statement: Not applicable.
Acknowledgments: Medical writing services were provided by Sidrah Rahman, MSc, of Adelphi Communications Ltd (Macclesfield, UK) and funded by Bayer HealthCare Pharmaceuticals, US, in accordance with Good Publication Practice.
Citation: Dato R, McKay RR. Darolutamide for the management of metastatic hormone-sensitive prostate cancer: a urologist-oncologist perspective. Rev Urol. 2024;23(3):exxx.
Corresponding author: Paul Dato, MD, Genesis Healthcare Partners, 3444 Kearny Villa Rd, Ste 201, San Diego, CA 92123
(paul.dato@uniohp.com)