Introduction
Non–muscle-invasive bladder cancer (NMIBC) accounts for a large proportion of bladder cancer cases and presents a substantial health and financial burden on patients and the health care system.1,2 Approximately 75% of patients with bladder cancer have NMIBC at diagnosis, and approximately 62 000 patients will be diagnosed with NMIBC in 2024, many of whom will develop recurrent disease, experience substantial emotional burdens, and have poor health-related quality of life (HRQOL).3,4 Non–muscle-invasive bladder cancer is stratified into low-risk, intermediate-risk, and high-risk categories, with an estimated 25% of newly diagnosed NMIBC cases classified as high risk.5-7 Patients with newly diagnosed high-risk NMIBC have a 60% to 70% chance of recurrence and a 10% to 45% chance of progression to muscle-invasive or metastatic bladder cancer within 5 years.8,9
Summary of Main Points
- NMIBC places a substantial health and economic burden on patients and the health care system.
- Patients with NMIBC have a variety of unmet treatment needs, such as lower risk of disease progression, improved symptom relief, increased HRQOL, and lessened discomfort and inconvenience of treatment administration.
- Appropriate use of current interventions such as TURBT, BCG, and chemotherapy can help address these challenges for patients.
- Future treatments may increase adherence, reduce disease recurrence, and reduce the need for TURBT, but cost-effectiveness should also be considered.
- Targeted education, counseling, and shared decision- making can better align treatment goals, increasing acceptance of and adherence to therapy.
Abbreviations
CIS carcinoma in situ
HRQOL health-related quality of life
NMIBC non–muscle-invasive bladder cancer
RFS recurrence-free survival
SWOG Southwest Oncology Group
TURBT transurethral resection of a bladder tumor
Regrettably, patients first diagnosed with NMIBC that advances to muscle-invasive disease often face a poorer prognosis than patients who initially exhibit muscle-
invasive disease.10-12 Interestingly, patients with NMIBC have reported a higher disease symptom burden than patients with more advanced stages of bladder cancer.13
Patients face an unmet need for safer and more effective treatments that lower the risk of disease progression, lessen the discomfort and inconvenience related to treatment administration, offer symptom relief, and increase HRQOL, especially for individuals with high-risk NMIBC. In a recent cross-sectional survey, patients with high-risk NMIBC reported that their top treatment goals are (1) to be cured or to prevent cancer recurrence and (2) to avoid the need for radical cystectomy.14 Therefore, clinicians should seek to better align current clinical practices with patients’ preferences and goals where possible, which may involve shared decision-making discussions with patients and their caregivers. This review discusses unmet needs and challenges related to the management of NMIBC from the patient’s perspective as well as current and future potential solutions.
Addressing Patient Needs in NMIBC
An accurate disease risk assessment by the urologist can optimize treatment strategies and enhance communication about disease progression and recurrence expectations. Of note, the definition of high-risk NMIBC differs slightly between current clinical practice guidelines or recommendations (eg, the American Urological Association, the European Association of Urology, the International Bladder Cancer Group), highlighting the importance of considering local treatment protocols and availability.5,15,16
Significance of a Primary Transurethral Resection of Bladder Tumor
Primary transurethral resection of bladder tumor (TURBT) is among the most common urologic procedures and may be the most critical intervention for the management of NMIBC. Prompt TURBT after flexible cystoscopy is essential because patients are most psychologically affected during the interval between finding a bladder mass during cystoscopy and tumor resection at TURBT. Psychological support and prompt TURBT after finding a bladder mass can improve the mental health of patients with NMIBC.17
Transurethral resection of bladder tumor can be challenging to perform well and may achieve differing results; the quality of surgical resection may strongly affect the risk of intravesical recurrence. Data from European trials involving nearly 2500 patients with NMIBC across 63 hospitals showed that early intravesical recurrence rates ranged from 0% to 43%, depending on the treatment location.18 These differences persisted after disease-related and treatment-related factors were accounted for, and they were thought to be explained by variations in TURBT quality. Strong evidence suggests that a more complete resection is associated with improved NMIBC outcomes and that some patients receive grossly incomplete TURBTs.18-21
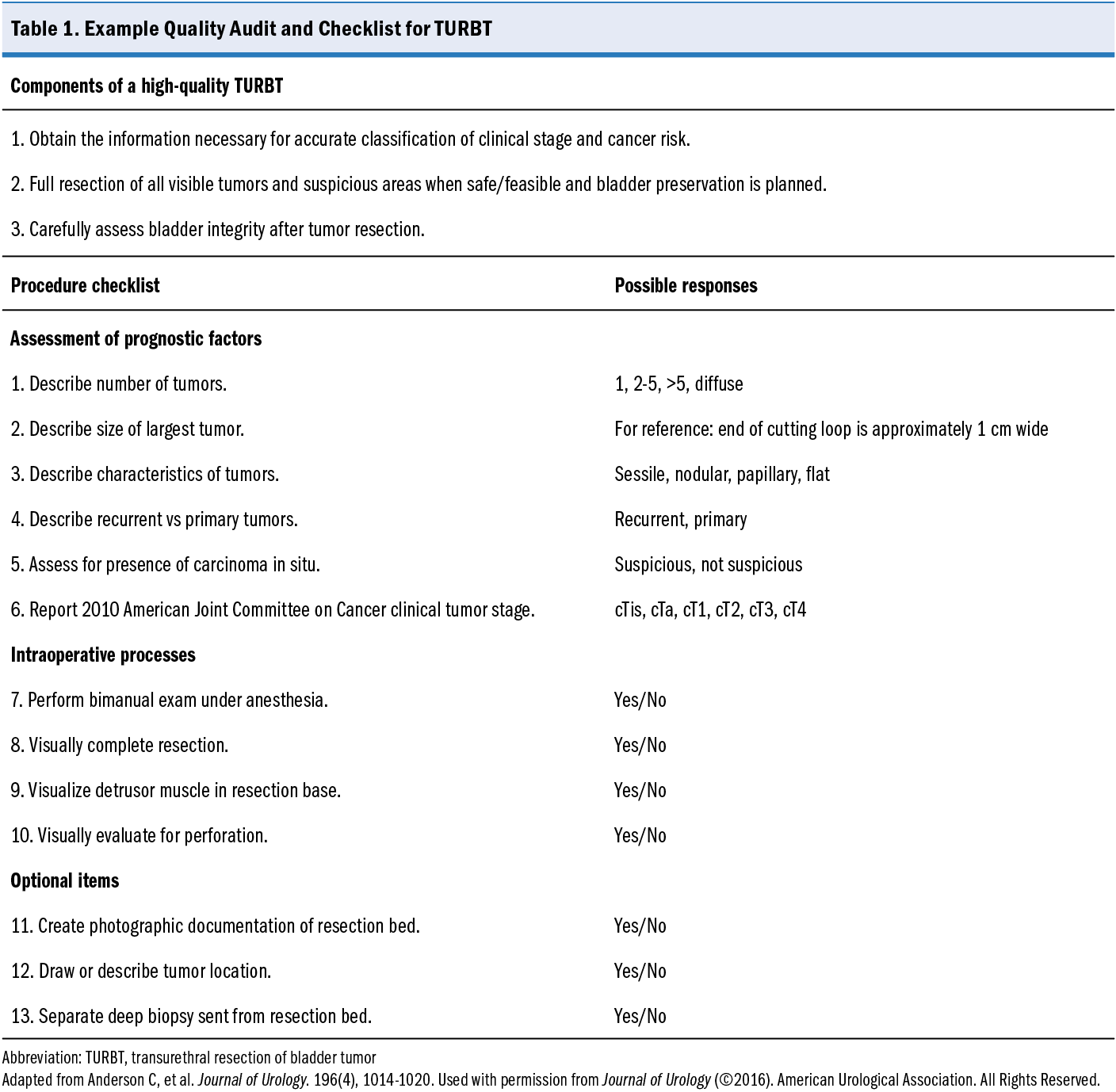
Standardized operative reports are a potential mechanism to assess and increase the quality of TURBT procedures. Standardizing the procedure documentation enhances communication of intraoperative findings, improves patient risk stratification, and fosters clearer dialogue with patients. Using a 10-item checklist during TURBT improved the reporting of critical procedural elements in a published study (Table 1).22 Although there was no apparent impact on the inclusion of muscle in the specimen, the use of a checklist or other standardized process may increase surgeon attention to essential aspects of the procedure and be a lever for quality improvement.
Significance of a repeat TURBT
Residual tumors can be found at the time of repeat resection in up to 50% of patients with high-grade Ta disease, with up to 15% of such tumors being upstaged.16 High-risk tumors, such as those that are larger and with multiple foci, have a high risk of incomplete initial resection. This incomplete resection substantially contributes to tumors that are inadequately treated and later identified as early recurrences.23 American Urological Association guidelines suggest repeat resection for most high-grade lesions, except for small high-grade Ta lesions where an initial visually complete resection was achieved.16
Patient Counseling When Offering Intravesical, Systemic, and Alternative Therapies
When selecting treatments for NMIBC, clinicians should involve patients in the decision-making process and provide appropriate counseling so that patients can make informed decisions. Patient considerations for BCG, chemotherapy, and other interventions are discussed in the sections that follow. For patients with low-grade urothelium-confined (Ta stage) NMIBC, deintensified strategies such as active surveillance, chemoablation, and office fulguration are valid and sometimes preferred over TURBT.1 A recent collaborative International Bladder Cancer Group review suggests that these less intensive strategies may result in lower rates of complications, less morbidity, lower health care costs, and better HRQOL without compromising oncologic safety for carefully selected patients with recurrent low-grade Ta NMIBC.1
BCG and the Patient Perspective
BCG is the gold-standard adjuvant therapy for high-risk NMIBC after TURBT.16 In an extensive analysis, BCG was shown to be superior in preventing recurrence (3 trials; relative risk, 0.56 [95% CI, 0.43-0.71]; I2 = 0%) and progression (4 trials; relative risk, 0.39 [95% CI, 0.24-0.64]; I2 = 40%) compared with no intravesical therapy.24 When tested against adjuvant single-agent chemotherapy regimens such as mitomycin and doxorubicin, BCG has superior prevention of recurrence and progression.25,26 Nonetheless, most patients with high-risk NMIBC receiving intravesical BCG do not achieve lasting remission; approximately 60% experience recurrence within 12 months, and about 80% experience recurrence within 5 years.8
Patients should be advised of the need for ongoing BCG maintenance therapy because clinical benefit and better recurrence rates are linked to BCG maintenance therapy. Southwest Oncology Group (SWOG) 8507 showed that maintenance BCG given as a weekly instillation for 3 weeks at months 3, 6, 12, 18, 24, 30, and 36, compared with induction BCG alone, increased the 5-year recurrence-free survival (RFS) from 41% to 60% (P < .0001).27 European Organisation for Research and Treatment of Cancer 30692, using a regimen similar to that of SWOG, showed that patients at high risk (high grade, T1) receiving 3 years of full-dose maintenance had a higher likelihood of remaining disease free at 5 years than patients receiving 1 year of maintenance (hazard ratio, 1.61 [95% CI, 1.13-2.30]; P = .009).28 Furthermore, several meta-analyses have reported that the superiority of BCG compared with intravesical chemotherapy to prevent recurrence and progression of high-risk NMIBC is limited to patients who receive maintenance therapy.26,29,30
Despite the clear need for ongoing maintenance BCG therapy, data indicate that many patients with high-risk NMIBC do not receive maintenance treatment according to established guidelines for a variety of reasons. In a post hoc analysis of patients with high-risk NMIBC treated with BCG, most (87%) who completed BCG maintenance therapy experienced local adverse reactions to treatment, with only 42% completing their full course of treatment.31 In a retrospective review of 729 patients receiving induction therapy, of whom 63% received BCG, only 10% of those starting on the SWOG maintenance protocol completed all 21 treatments, and 55% completed a monthly protocol of 9 treatments.32 Another study followed 411 patients treated with BCG for a year and found that only 52.3% of patients completed the recommended 1-year course of BCG.33
Despite its benefits, intravesical BCG has several challenges. It has been associated with lower HRQOL during treatment, and local (bladder) and systemic side effects are common (eg, fatigue, flulike symptoms, diarrhea).7,34 Major adverse events from BCG induction therapy remain low; nevertheless, irritative lower urinary tract symptoms, such as hematuria, dysuria, urinary urgency or frequency, and urinary tract infections, are common and can make BCG treatment adherence difficult.35
In a cross-sectional online survey of 171 US adults with NMIBC and no other cancer, patients reported a high disease symptom burden of NMIBC, which has also been shown to hinder treatment completion.36 The emotional well-being subscale presented the greatest burden in this study, with nearly half of the participants fearing that their condition would worsen. Of the physical symptoms and treatment side effects, participants most commonly reported trouble sleeping and a lack of energy.36 These results are in line with a recent systematic review of HRQOL in NMIBC that identified worry about future disease, fatigue, and insomnia as the most troubling symptoms patients experience during intravesical therapy.34
Patients need thorough counseling on expectations and adverse event management related to BCG therapy to increase the likelihood of successful treatment. Patients with NMIBC often have a poor perception of disease control and believe that their disease will continue over a prolonged period; this belief is particularly notable in older adults.17 Clearly reviewing expectations and key points of BCG therapy beforehand may help increase patients’ acceptance of and adherence to the full treatment course.
Chemotherapy (Gemcitabine and Docetaxel) as an Alternative to BCG for High-Risk NMIBC
The main challenge with BCG is that persistent production shortages have resulted in urological practices lacking a practical, readily available first-line treatment. In 2015, sequential intravesical gemcitabine and docetaxel was initially described as an effective and well-tolerated therapy after BCG failure, with 2-year RFS of 34% to 46%.37 A recent retrospective cohort study of 312 patients with treatment-naive NMIBC showed that gemcitabine and docetaxel were associated with better high-grade RFS (hazard ratio, 0.57 [95% CI, 0.33-0.97]; P = .04) and RFS (hazard ratio, 0.56 [95% CI, 0.34-0.92]; P = .02) than treatment with BCG. Induction therapy with BCG was associated with more frequent treatment discontinuation than induction therapy with gemcitabine and docetaxel (9.2% vs 2.9%; P = .02).37 In patients with high-risk NMIBC, gemcitabine and docetaxel therapy may result in lower rates of high-grade disease recurrence and treatment discontinuation than BCG therapy; further studies are warranted.
Timing of Transition to Radical Surgery for BCG-Naive Patients
Initial radical surgery is rarely needed in treatment-naive high-risk NMIBC. American Urological Association guidelines recommend that for patients with high-grade T1 disease with associated carcinoma in situ (CIS), lymphovascular invasion, or variant histology, a clinician should consider offering an initial radical cystectomy.16 Evidence of the effectiveness of intravesical therapy for patients with non–muscle invasive urothelial carcinoma featuring variant histology is limited.
Alternative Therapies for NMIBC
Trimodal therapy consists of TURBT followed by external beam radiation therapy and concurrent chemotherapy.38 Ideal candidates for trimodal therapy include patients with unifocal cT2 disease without hydronephrosis or concomitant multifocal CIS. Patients with CIS (and other NMIBC) should be encouraged to enroll in clinical trials.
Partial cystectomy typically is considered only for carefully selected patients with NMIBC who have small, solitary tumors amenable to resection with adequate margins (eg, in the diverticulum, with no concomitant CIS or histologic subtype).39 Before partial cystectomy, random bladder or directed biopsies with blue light cystoscopy should be conducted as well as prostatic urethral biopsies to rule out concomitant CIS.
Financial Toxicity to Patients With NMIBC
Non–muscle-invasive bladder cancer poses a substantial challenge both for patients and for health care systems. Although data on the financial impact on patients are limited, the current treatment options present various challenges depending on the patient’s circumstances. Employed patients indicated a high impact on work productivity from NMIBC, reporting a 59% overall productivity loss.32 This high impact is consistent with that of uncomplicated urinary tract infections (56%) and exceeds that of non–dialysis-dependent chronic kidney disease (22%).32 A prior prospective study observed a low prevalence of financial toxicity among patients with NMIBC; specifically, in that study, 4% to 16% of patients experienced some degree of NMIBC-related financial strain, with approximately 5% reporting a substantial economic burden due to NMIBC treatment at 2-year follow-up.40 This survey, however, was not exclusive to intravesical therapy, and only 58% of patients surveyed had received treatment within the past year, potentially reducing the perceived impact of treatment. The unusually low financial strain reported also may be attributed to many patients being retired, homemakers, or unemployed and not looking for work.41 Ahlschlager et al42 reported more days of absenteeism after bladder-sparing treatment than after cystectomy, probably because of the frequent clinic visits needed for intravesical therapy compared with the single procedure performed for cystectomy.
Taken together, these findings underscore that patients with NMIBC that was treated with intravesical therapy within the past year experience a substantial impact on HRQOL and that novel intravesical treatments with greater patient tolerability are warranted. Although the advent of newer agents may improve outcomes and HRQOL for certain patients with NMIBC, these agents typically have higher costs, increasing the overall costs of NMIBC treatment. Thus, the potential benefit of newer agents should be balanced against the higher costs of therapy. Clinicians should weigh the implications for patients and health care systems of selecting cost-effective regimens and consider existing therapies (eg, BCG) and inexpensive alternatives (gemcitabine and docetaxel) for certain patients. As part of shared decision-making with patients, clinicians might also discuss indirect costs, or social costs, of various treatments, including the need for frequent visits or follow up and the duration of therapy.
Addressing Unmet Treatment Needs in NMIBC
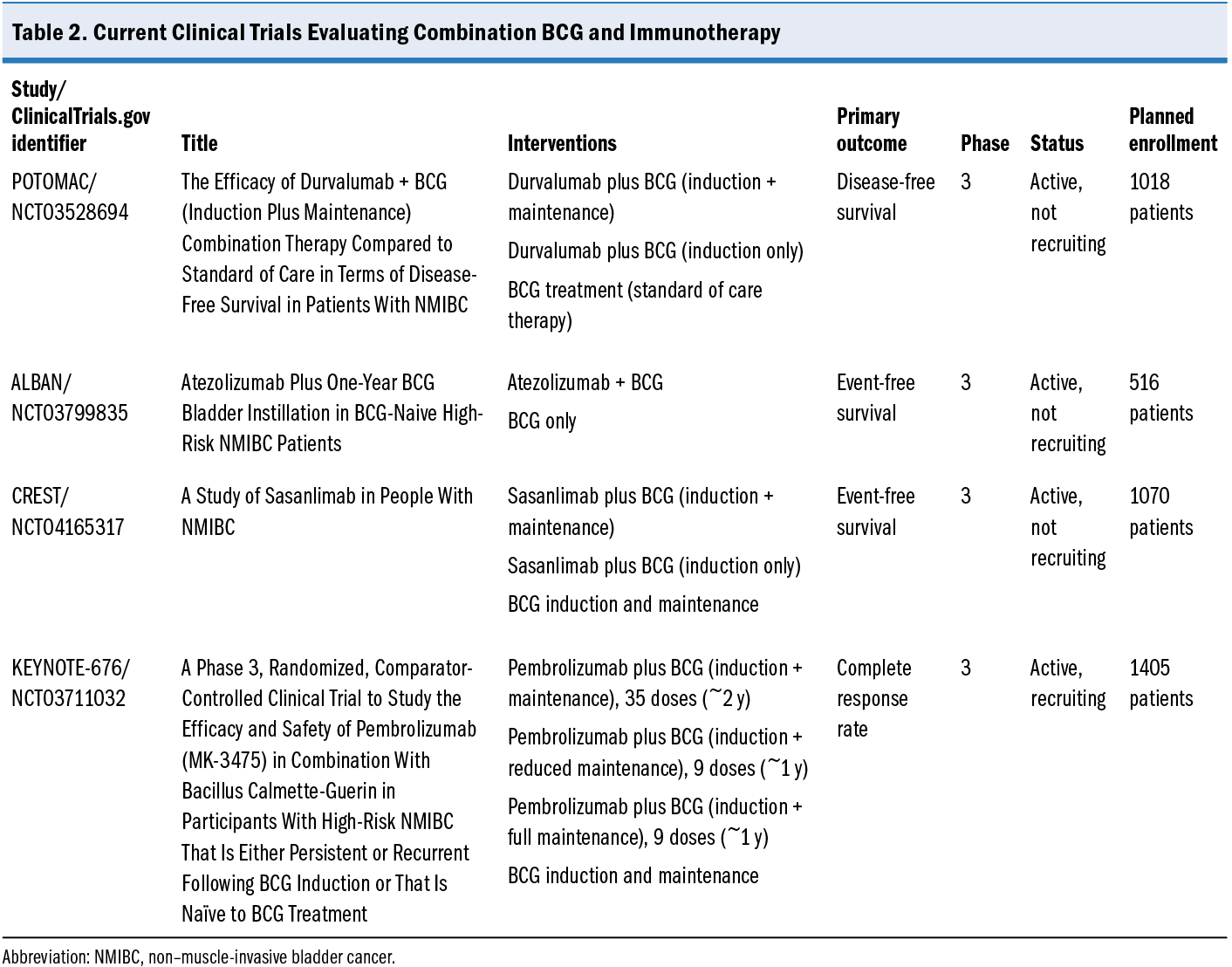
Several potential therapeutic regimens being assessed in clinical trials may help address unmet needs in NMIBC. Treatment options that increase treatment adherence, reduce disease recurrence, or avoid or postpone TURBT are likely to have a substantial impact on NMIBC management. Ongoing trials for NMIBC include evaluations related to intravesical drug delivery systems, BCG and chemotherapy, and BCG with immunotherapy.
A Study of TAR-200 in Combination With Cetrelimab or TAR-200 Alone Versus Intravesical Bacillus Calmette-Guérin (BCG) in Participants With BCG-naïve High-risk Non-muscle Invasive Bladder Cancer (HR-NMIBC)—the SunRISe-3 trial (ClinicalTrials.gov identifier NCT05714202)—is investigating the efficacy of TAR-200, an intravesical drug delivery system that provides local continuous gemcitabine release within the bladder, either alone or combined with cetrelimab, against BCG in treating patients with BCG-naive bladder cancer. The Intravesical BCG vs GEMDOCE in NMIBC (BRIDGE) study (ClinicalTrials.gov identifier NCT05538663) is evaluating whether event-free survival is noninferior for gemcitabine and docetaxel compared with standard BCG for patients with BCG-naive high-grade NMIBC. The study will randomly assign 870 patients 1:1 (stratified by pure CIS, pure papillary, and mixed) to gemcitabine and docetaxel 6 weekly cycles with monthly maintenance vs BCG 6 weekly cycles with SWOG protocol maintenance. The primary outcome is event-free survival, defined as the time from random assignment to high-grade recurrence in the bladder (CIS, high-grade Ta, high-grade T1 or T2 disease), disease progression, or death. Several trials are assessing BCG plus immunotherapy for NMIBC (Efficacy and Safety of Pembrolizumab [MK-3475] in Combination With Bacillus Calmette-Guerin [BCG] in High-Risk Non-Muscle Invasive Bladder Cancer [HR NMIBC[ [KEYNOTE-676; ClinicalTrials.gov identifier NCT03711032]; A Study of Sasanlimab in People With Non-muscle Invasive Bladder Cancer [CREST; ClinicalTrials.gov identifier NCT04165317]; Assessment of Efficacy and Safety of Durvalumab Plus BCG Compared to the Standard Therapy With BCG in Non-muscle Invasive Bladder Cancer [POTOMAC; ClinicalTrials.gov identifier NCT03528694]; Atezolizumab Plus One-year BCG Bladder Instillation in BCG-naive High-risk Non-muscle Invasive Bladder Cancer Patients [ALBAN; ClinicalTrials.gov identifier NCT03799835]), and details are reviewed in Table 2.
Future Directions to Increase Patient Satisfaction
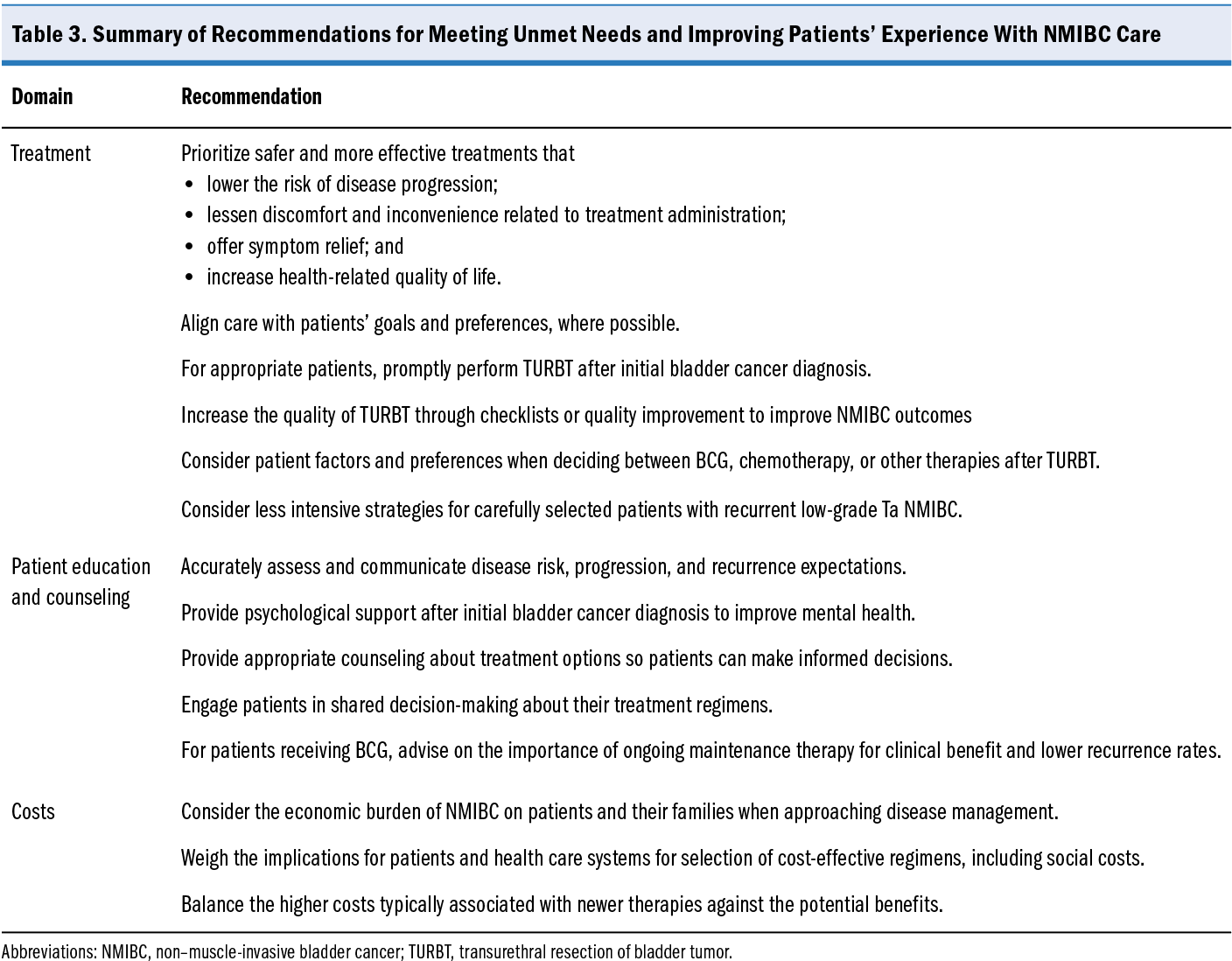
Future directions to improve patients’ experience and satisfaction with NMIBC treatment can be facilitated by clinicians in some cases. Although practicing clinicians may have limited impact on developing better treatment options that have fewer side effects, improving patient education, resource allocation, and supportive care can improve outcomes (Table 3).
Identifying the subset of patients who are (or are at risk of) experiencing financial toxicity can help guide health care policy in targeting the allocation of resources (eg, subsidies) to patients who need the most support. Pending approvals for the use of novel therapies may substantially raise the cost of treatment, increasing the need for careful allocation of resources. In 1 study, most patients reported that they stopped BCG treatment because of logistical challenges (eg, inability to get to the hospital or clinic), negative perceptions about the BCG procedure, efficacy, safety, or other reasons.14 Focusing on patient education about treatment options and instrumental social support to facilitate treatment access and potentially prevent early treatment discontinuation may improve care for patients with high-risk NMIBC. This potential is aligned with prior research, which has described high informational needs for patients with NMIBC regarding their cancer diagnosis, side effects of treatments, and expected pace of recovery as well as unmet supportive care needs that include emotional support, desire for shared decision-making, and help managing symptoms.43
clinicians may have limited impact on developing better treatment options that have fewer side effects, improving patient education, resource allocation, and supportive care can improve outcomes (Table 3).
Identifying the subset of patients who are (or are at risk of) experiencing financial toxicity can help guide health care policy in targeting the allocation of resources (eg, subsidies) to patients who need the most support. Pending approvals for the use of novel therapies may substantially raise the cost of treatment, increasing the need for careful allocation of resources. In 1 study, most patients reported that they stopped BCG treatment because of logistical challenges (eg, inability to get to the hospital or clinic), negative perceptions about the BCG procedure, efficacy, safety, or other reasons.14 Focusing on patient education about treatment options and instrumental social support to facilitate treatment access and potentially prevent early treatment discontinuation may improve care for patients with high-risk NMIBC. This potential is aligned with prior research, which has described high informational needs for patients with NMIBC regarding their cancer diagnosis, side effects of treatments, and expected pace of recovery as well as unmet supportive care needs that include emotional support, desire for shared decision-making, and help managing symptoms.43
Conclusion
The substantial burden of NMIBC on patients and the health care system warrants attention to potential solutions to challenges faced when managing NMIBC. Unmet treatment needs for patients with NMIBC include lower risk of disease progression, improved symptom relief, increased HRQOL, and lessened discomfort and inconvenience of treatment administration. Appropriate use of current interventions such as TURBT, BCG, and chemotherapy can help address these challenges for patients, along with appropriate education, counseling, and shared decision-making. Financial toxicities to patients and the health care system should also be considered, and cost-effective approaches based on careful patient and treatment selection can help appropriately allocate resources. Future treatments that may improve treatment adherence, reduce disease recurrence, or avoid or postpone TURBT could have a substantial impact on the care of patients with NMIBC.
References
1. Contieri R, Soloway MS, Gontero P, et al. Deintensification of treatment for low-grade bladder tumors: a collaborative review by the International Bladder Cancer Group (IBCG). Eur Urol Oncol. 2024:S258893112400186X. doi:10.1016/j.euo.2024.08.001
2. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-263. doi:10.3322/caac.21834
3. National Cancer Institute. Cancer of the urinary bladder: cancer stat facts. SEER. Accessed October 28, 2024. https://seer.cancer.gov/statfacts/html/urinb.html
4. Grabe-Heyne K, Henne C, Mariappan P, Geiges G, Pöhlmann J, Pollock RF. Intermediate and high-risk non–muscle-invasive bladder cancer: an overview of epidemiology, burden, and unmet needs. Front Oncol. 2023;13:1170124. doi:10.3389/fonc.2023.1170124
5. Babjuk M, Burger M, Capoun O, et al. European Association of Urology guidelines on non–muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ). Eur Urol. 2022;81(1):75-94. doi:10.1016/j.eururo.2021.08.010
6. Cavaliere C, D’Aniello C, Cecere S, et al. Non muscle invasive bladder cancer treatment. World Cancer Research Journal. 2014;1(1):e126.
7. Williams SB, Howard LE, Foster ML, et al. Estimated costs and long-term outcomes of patients with high-risk non–muscle-invasive bladder cancer treated with bacillus Calmette-Guérin in the Veterans Affairs Health System. JAMA Netw Open. 2021;4(3):e213800. doi:10.1001/jamanetworkopen.2021.3800
8. Sylvester RJ, Van Der Meijden APM, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49(3):466-477. doi:10.1016/j.eururo.2005.12.031
9. Jeong SH, Han JH, Jeong CW, et al. Clinical determinants of recurrence in pTa bladder cancer following transurethral resection of bladder tumor. BMC Cancer. 2022;22(1):631. doi:10.1186/s12885-022-09733-8
10. Schrier BP, Hollander MP, Van Rhijn BWG, Kiemeney LALM, Witjes JA. Prognosis of muscle-invasive bladder cancer: difference between primary and progressive tumours and implications for therapy. Eur Urol. 2004;45(3):292-296. doi:10.1016/j.eururo.2003.10.006
11. Breau RH, Karnes RJ, Farmer SA, et al. Progression to detrusor muscle invasion during urothelial carcinoma surveillance is associated with poor prognosis. BJU Int. 2014;113(6):900-906. doi:10.1111/bju.12403
12. Van Den Bosch S, Witjes JA. Long-term cancer-specific survival in patients with high-risk, non–muscle-invasive bladder cancer and tumour progression: a systematic review. Eur Urol. 2011;60(3):493-500. doi:10.1016/j.eururo.2011.05.045
13. Cella D, Rosenbloom SK, Beaumont JL, et al. Development and validation of 11 symptom indexes to evaluate response to chemotherapy for advanced cancer. J Natl Compr Canc Netw. 2011;9(3):268-278. doi:10.6004/jnccn.2011.0026
14. Kopenhafer L, Thompson A, Chang J, et al. Patient experience and unmet needs in high-risk nonmuscle-invasive bladder cancer: insights from qualitative interviews and a cross-sectional survey. Urol Oncol. 2024;42(3):70.e1-70.e10. doi:10.1016/j.urolonc.2024.01.013
15. Li R, Hensley PJ, Gupta S, et al. Bladder-sparing therapy for bacillus Calmette-Guérin–unresponsive non–muscle-invasive bladder cancer: International Bladder Cancer Group recommendations for optimal sequencing and patient selection. Eur Urol. 2024:S0302283824025168. doi:10.1016/j.eururo.2024.08.001
16. Holzbeierlein JM, Bixler BR, Buckley DI, et al. Diagnosis and treatment of non–muscle invasive bladder cancer: AUA/SUO guideline: 2024 amendment. J Urol. 2024;211(4):533-538. doi:10.1097/JU.0000000000003846
17. Tan WS, Teo CH, Chan D, et al. Exploring patients’ experience and perception of being diagnosed with bladder cancer: a mixed‐methods approach. BJU Int. 2020;125(5):669-678. doi:10.1111/bju.15008
18. Brausi M, Collette L, Kurth K, et al. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies. Eur Urol. 2002;41(5):523-531. doi:10.1016/S0302-2838(02)00068-4
19. Mariappan P, Finney SM, Head E, et al. Good quality white‐light transurethral resection of bladder tumours (GQ‐WLTURBT) with experienced surgeons performing complete resections and obtaining detrusor muscle reduces early recurrence in new non‐muscle‐invasive bladder cancer: validation across time and place and recommendation for benchmarking. BJU Int. 2012;109(11):1666-1673. doi:10.1111/j.1464-410X.2011.10571.x
20. Sfakianos JP, Kim PH, Hakimi AA, Herr HW. The effect of restaging transurethral resection on recurrence and progression rates in patients with nonmuscle invasive bladder cancer treated with intravesical bacillus Calmette-Guérin. J Urol. 2014;191(2):341-345. doi:10.1016/j.juro.2013.08.022
21. Adiyat KT, Katkoori D, Soloway CT, De Los Santos R, Manoharan M, Soloway MS. “Complete transurethral resection of bladder tumor”: are the guidelines being followed? Urology. 2010;75(2):365-367. doi:10.1016/j.urology.2009.08.082
22. Anderson C, Weber R, Patel D, et al. A 10-item checklist improves reporting of critical procedural elements during transurethral resection of bladder tumor. J Urol. 2016;196(4):1014-1020. doi:10.1016/j.juro.2016.03.151
23. Di̇vri̇k RT, Yildirim Ü, Zorlu F, Özen H. The effect of repeat transurethral resection on recurrence and progression rates in patients with T1 tumors of the bladder who received intravesical mitomycin: a prospective, randomized clinical trial. J Urol. 2006;175(5):1641-1644. doi:10.1016/S0022-5347(05)01002-5
24. Chou R, Buckley D, Fu R, et al. Emerging approaches to diagnosis and treatment of non–muscle-invasive bladder cancer. Comparative effectiveness review no. 153. (Prepared by the Pacific Northwest Evidence-based Practice Center under Contract No. 290-2012-00014-I.) AHRQ publication no. 15(16)-EHC017-EF. Agency for Healthcare Research and Quality. October 2015; addendum November 2016. www.effectivehealthcare.ahrq.gov/reports/final.cfm
25. Shelley MD, Wilt TJ, Court J, Coles B, Kynaston H, Mason MD. Intravesical bacillus Calmette‐Guérin is superior to mitomycin C in reducing tumour recurrence in high‐risk superficial bladder cancer: a meta‐analysis of randomized trials. BJU Int. 2004;93(4):485-490. doi:10.1111/j.1464-410X.2003.04655.x
26. Malmström PU, Sylvester RJ, Crawford DE, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non–muscle-invasive bladder cancer. Eur Urol. 2009;56(2):247-256. doi:10.1016/j.eururo.2009.04.038
27. Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000;163(4):1124-1129. doi:10.1016/s0022-5347(05)67707-5
28. Oddens J, Brausi M, Sylvester R, et al. Final results of an EORTC-GU Cancers Group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol. 2013;63(3):462-472. doi:10.1016/j.eururo.2012.10.039
29. Sylvester RJ, Oosterlinck W, Witjes JA. The schedule and duration of intravesical chemotherapy in patients with non–muscle-invasive bladder cancer: a systematic review of the published results of randomized clinical trials. Eur Urol. 2008;53(4):709-719. doi:10.1016/j.eururo.2008.01.015
30. Sylvester RJ, Van Der Meijden APM, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168(5):1964-1970. doi:10.1016/S0022-5347(05)64273-5
31. Miyazaki J, Hinotsu S, Ishizuka N, et al. Adverse reactions related to treatment compliance during BCG maintenance therapy for non–muscle-invasive bladder cancer. Jpn J Clin Oncol. 2013;43(8):827-834. doi:10.1093/jjco/hyt086
32. Tapiero S, Helfand A, Kedar D, et al. Patient compliance with maintenance intravesical therapy for nonmuscle invasive bladder cancer. Urology. 2018;118:107-113. doi:10.1016/j.urology.2018.04.039
33. Serretta V, Scalici Gesolfo C, Alonge V, Cicero G, Moschini M, Colombo R. Does the compliance to intravesical BCG differ between common clinical practice and international multicentric trials? Urol Int. 2016;96(1):20-24. doi:10.1159/000430501
34. Lee LJ, Kwon CS, Forsythe A, Mamolo CM, Masters ET, Jacobs IA. Humanistic and economic burden of non–muscle invasive bladder cancer: results of two systematic literature reviews. Clinicoecon Outcomes Res. 2020;12:693-709. doi:10.2147/CEOR.S274951
35. Steinberg RL, Thomas LJ, Mott SL, O’Donnell MA. Multi-perspective tolerance evaluation of bacillus Calmette-Guerin with interferon in the treatment of non–muscle invasive bladder cancer. Bladder Cancer. 2019;5(1):39-49. doi:10.3233/BLC-180203
36. Totev TI, Ireland A, Shah A, Tardif-Samson A, Lefebvre P, Pilon D. Overall burden and impact on health-related quality of life associated with intravesical treatment of patients with non–muscle invasive bladder cancer in the United States. Curr Med Res Opin. 2024;40(11):2003-2011. doi:10.1080/03007995.2024.2411424
37. McElree IM, Steinberg RL, Mott SL, O’Donnell MA, Packiam VT. Comparison of sequential intravesical gemcitabine and docetaxel vs bacillus Calmette-Guérin for the treatment of patients with high-risk non–muscle-invasive bladder cancer. JAMA Netw Open. 2023;6(2):e230849. doi:10.1001/jamanetworkopen.2023.0849
38. Pham A, Ballas LK. Trimodality therapy for bladder cancer: modern management and future directions. Curr Opin Urol. 2019;29(3):210-215. doi:10.1097/MOU.0000000000000601
39. Lu Y, Jiang S, Yin X, et al. Long-term effect of transurethral partial cystectomy with a 2-micrometer continuous-wave laser for non–muscle-invasive bladder cancer. Front Surg. 2023;10:1117997. doi:10.3389/fsurg.2023.1117997
40. Sharma V, Fero KE, Lec PM, et al. PD25-03. The incidence and predictors of financial toxicity in a prospective cohort of patients with non–muscle invasive bladder cancer. J Urol. 2021;206(suppl 3):e432. doi:10.1097/JU.0000000000002018.03
41. Sanchez A, Agarwal N. Quantifying the costs of care among patients with high-risk non–muscle-invasive bladder cancer treated in the Veterans Health Administration. JAMA Netw Open. 2021;4(3):e213816. doi:10.1001/jamanetworkopen.2021.3816
42. Ahlschlager L, McCabe S, Deal AM, et al. The effect of treatment on work productivity in patients with bladder cancer. Urol Oncol. 2023;41(6):293.e15-293.e21. doi:10.1016/j.urolonc.2023.01.020
43. Chung J, Kulkarni GS, Morash R, et al. Assessment of quality of life, information, and supportive care needs in patients with muscle and non–muscle invasive bladder cancer across the illness trajectory. Support Care Cancer. 2019;27(10):3877-3885. doi:10.1007/s00520-019-4649-z
Article Information
Published: December 13, 2024.
Conflict of Interest Disclosures: Jason Hafron is a consultant for Janssen Biotech Inc and Pfizer and a meeting participant and lecturer for Janssen Biotech Inc, Merck & Co Inc, Pfizer, and AstraZeneca studies. Ashish Kamat has received grants or contracts from FKD Therapies (now Ferring), the Patient-Centered Outcomes Research Institute, Photocure, Seagen, EnGene, Arquer Diagnostics, and SWOG; is on the advisory board or has received consulting fees from Astellas Pharma, Biological Dynamics, Bristol-Myers Squibb, CG Oncology, Cystotech, Eisai, EnGene, Ferring, Imagin Medical, Imvax, Incyte, Janssen, Medac, Merck, Nonagen Bioscience, Pfizer, Photocure, Protara Therapeutics, Roche, Seagen, Sessen Bio, Theralase, Urogen Pharma, US Biotest, and Vivet Therapeutics; holds a patent for CyPRIT; and has played a leadership or fiduciary role for Euopean Urology Oncology, the International Bladder Cancer Group, theh International Bladder Cancer Network, the Journal of Urology, and UroToday. Both authors had the final responsibility for the decision to submit the article for publication.
Funding/Support: No funding was received for this article.
Author Contributions: Ashish Kamat and Jason Hafron participated in conceptualizing, reviewing, and editing the manuscript. Both authors read and approved the final article.
Data Availability Statement: No new data were generated for this article.
Acknowledgments: The authors thank Austin Ulrich, PharmD, BCACP, of Dragonfly Editorial for medical writing assistance in preparing the manuscript.