Prostate cancer (PCa) is the second-leading cause of cancer death among men in the United States. The American Cancer Society estimates that more than 299 010 men will be diagnosed with PCa in 2024, with more than 35 250 PCa-associated deaths.1 It is estimated that of those patients treated for localized PCa, 30% to 35% will experience biochemical recurrence (BCR), which further increases the risk of PCa death. Although multiple definitions have been proposed, BCR is generally present when a man has a persistently rising prostate-specific antigen (PSA) value after definitive local therapy (Table 1) in the absence of radiographic evidence of metastatic disease. This definition has evolved and will continue to develop, with advances in genomics, next-generation sequencing, and digital pathology.
With the emergence and success of novel hormone-based agents in advanced disease, a recent focus has been on extending that benefit to patients with PCa earlier in the treatment pathway. In June 2023, the Safety and Efficacy Study of Enzalutamide Plus Leuprolide in Patients With Nonmetastatic Prostate Cancer (EMBARK) study data were published, demonstrating increases in overall survival and metastasis-free survival in men with high-risk biochemically recurrent PCa treated with enzalutamide plus leuprolide or enzalutamide monotherapy.2 Both regimens resulted in substantially longer metastasis-free survival and longer time to PSA progression.
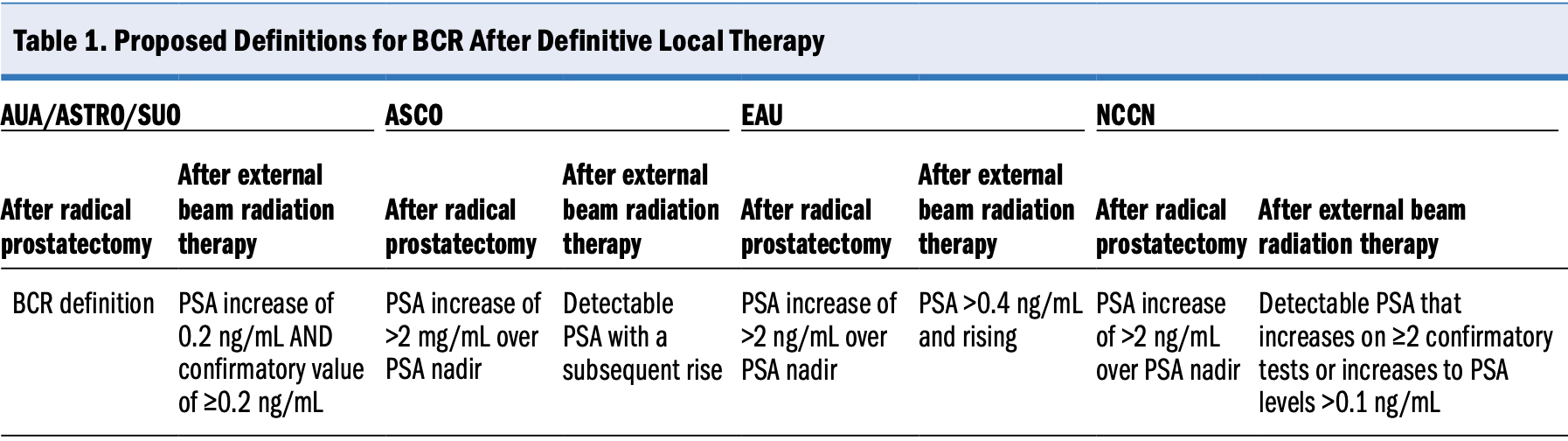
Abbreviations: ASCO, American Society of Clinical Oncology; ASTRO, American Society for Radiation Oncology; AUA, American Urological Association; BCR, biochemical recurrence; EAU, European Association of Urology; NCCN, National Comprehensive Cancer Network; PSA, prostate-specific antigen; SUO, Society of Urologic Oncology.
Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Prostate Cancer V.1.2023. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Available at www.NCCN.org. 2022.
In addition, patients receiving enzalutamide plus leuprolide experienced a longer period before receiving another antineoplastic therapy than patients on leuprolide alone did, and these patients were able to maintain an overall quality of life.3
From January to June 2024, Specialty Networks led a program sponsored by Astellas to educate the Specialty Networks network of community-based urology practices on the new indication for enzalutamide in patients with high-risk biochemically recurrent PCa. Specialty Networks identified 10 practices—some without a high-risk biochemically recurrent PCa focus and some with a formalized process to identify and treat patients with high-risk biochemically recurrent PCa. Specialty Networks interviewed health care professionals, advanced practice professionals, and patient navigators to assess how they diagnose, monitor, and treat patients with high-risk biochemically recurrent PCa. Specialty Networks evaluated the practices’ workflows, potential patient care gaps, and operational needs to organize educational material for the entire Specialty Networks membership.
Abbreviations
BCR biochemical recurrence
PCa prostate cancer
PSA prostate-specific antigen
After working with these practices and multiple key opinion leaders, Specialty Networks created a patient playbook that covers clinical and operational considerations, patient identification, patient journeys in the PCa BCR and high-risk BCR spaces, and additional resources. Now, as a clinician counterpoint to the patient playbook, the Localized to High-Risk Biochemically Recurrent Prostate Cancer Patient Playbook is designed to educate clinicians and ancillary staff on the diagnosis, monitoring, and treatment guidelines for patients with biochemically recurrent PCa (Figure 1). The operationally intensive nature of diagnosing, monitoring, and tracking PCa patient populations has led to a team-based approach to care that, over the years, has shown success in treating advanced PCa. During Specialty Networks’ conversations with urology practices, it became evident that the depth of knowledge and organization necessary to treat biochemically recurrent PCa varies. The goal of the clinician playbook is to improve practice ratios of patients receiving treatment for BCR by increasing clinical knowledge and using patient identification tools through PPS Analytics (Specialty Networks).
The Localized to High-Risk Biochemically Recurrent Prostate Cancer Patient Playbook contains links to the Specialty Networks advanced PCa guidelines and to the American Urological Association and National Comprehensive Cancer Network PCa guidelines. In addition, the material reviews patient selection for the new enzalutamide indication and provides tools for identifying these patients in clinics. Best practices are highlighted, from early diagnosis of PCa to management of posttherapy PSA rise.
The Specialty Networks team has educated their Prostate Cancer Navigator network on the Localized to High-Risk Biochemically Recurrent Prostate Cancer Patient Playbook by hosting monthly Navigator webinars and patient navigator meetings. In addition, this information has been disseminated to the entire Specialty Networks membership through email blasts and newsletters, and the link to the playbook is available on the Specialty Networks website.

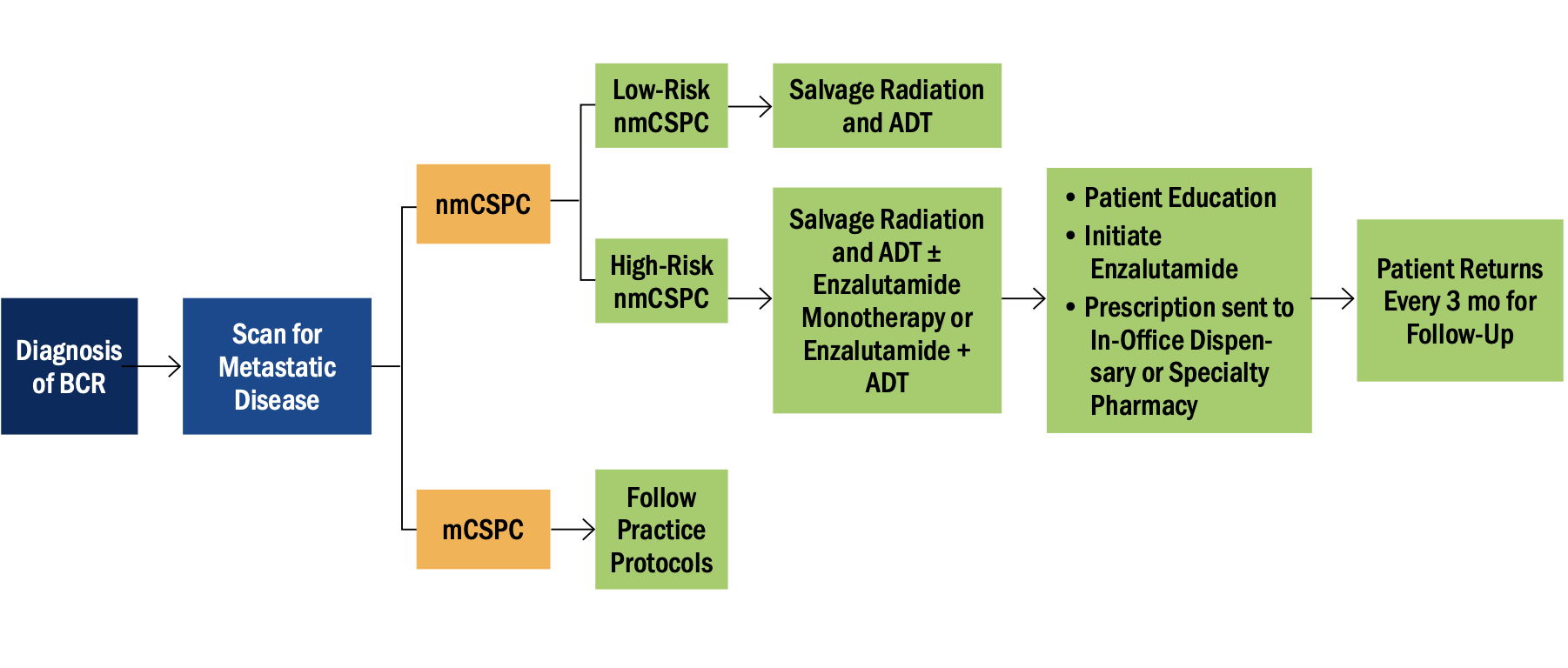
Figure 1. Patient journey for biochemically recurrent prostate cancer.
Abbreviations: ADT, androgen-deprivation therapy; BCR, biochemical recurrence; mCSPC, metastatic castration-sensitive prostate cancer; nmCSPC, nonmetastatic castration-sensitive prostate cancer.
References
1. Key statistics for prostate cancer. American Cancer Society. Updated January 19, 2024. Accessed September 10, 2024. https://www.cancer.org/cancer/types/prostate-cancer/about/key-statistics.html
2. LBA02-09 EMBARK: a phase 3 randomized study of enzalutamide or placebo plus leuprolide acetate and enzalutamide monotherapy in high-risk biochemically recurrent prostate cancer. J Urol. 2023;210(1):224-226. doi:10.1097/JU.0000000000003518
3. Freedland SJ, de Almeida Luz M, De Giorgi U, et al. Improved outcomes with enzalutamide in biochemically recurrent prostate cancer. N Engl J Med. 2023;389(16):1453-1465. doi:10.1056/NEJMoa2303974
Article Information
Published: September 13, 2024.
Conflict of Interest Disclosures: Dr Jayram is a consultant for J&J and a speaker for Bayer.
Funding/Support: Astellas sponsored this project with Specialty Networks.
Author Contributions: All authors contributed equally to this editorial.
Data Availability Statement: No new data were generated for this editorial. All data used are cited.
Citation: Jayram G, Grant K, Nalley J, Smith N. The Specialty Networks Localized to High-Risk Biochemically Recurrent Prostate Cancer Patient Playbook. Rev Urol. 2024;23(3):e89-e91.
Corresponding author: Gautam Jayram, MD, Advanced Therapeutics Center, Urology Associates, PC, 2801 Charlotte Ave, Nashville, TN 37215 (gtjayram@ua-pc.com)